Product Description
A novel CDH17-targeting antibody drug conjugate (ADC). CDH17 specific monoclonal antibodies were generated using traditional hybridoma technology and splenocytes isolated from mice immunized with cocktails of NIH3T3 cells overexpressing full length hCHD17 plus purified mammalian expressed hCDH17 extracellular domain protein (aa 23-787). Selective mAb binding was confirmed by flow cytometry and mAb internalization rate was assessed by immunofluorescence (IF). TORL-3-600 was generated from a fully humanized CDH17 mAb by MMAE conjugation with a cleavable linker. Cell membrane staining of CDH17 in patient tumor microarrays (TMAs), cell line (CDX) and patient derived xenografts (PDX) was evaluated by IHC assay. (Sourced from: https://aacrjournals.org/cancerres/article/84/6_Supplement/1900/740888/Abstract-1900-TORL-3-600-a-novel-antibody-drug)
Mechanisms of Action: CDH17 Inhibitor
Novel Mechanism: Yes
Modality: Antibody Drug Conjugate
Route of Administration: N/A
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: TORL Biotherapeutics
Company Location: Western America
Company CEO:
Additional Commercial Interests: None
Clinical Description
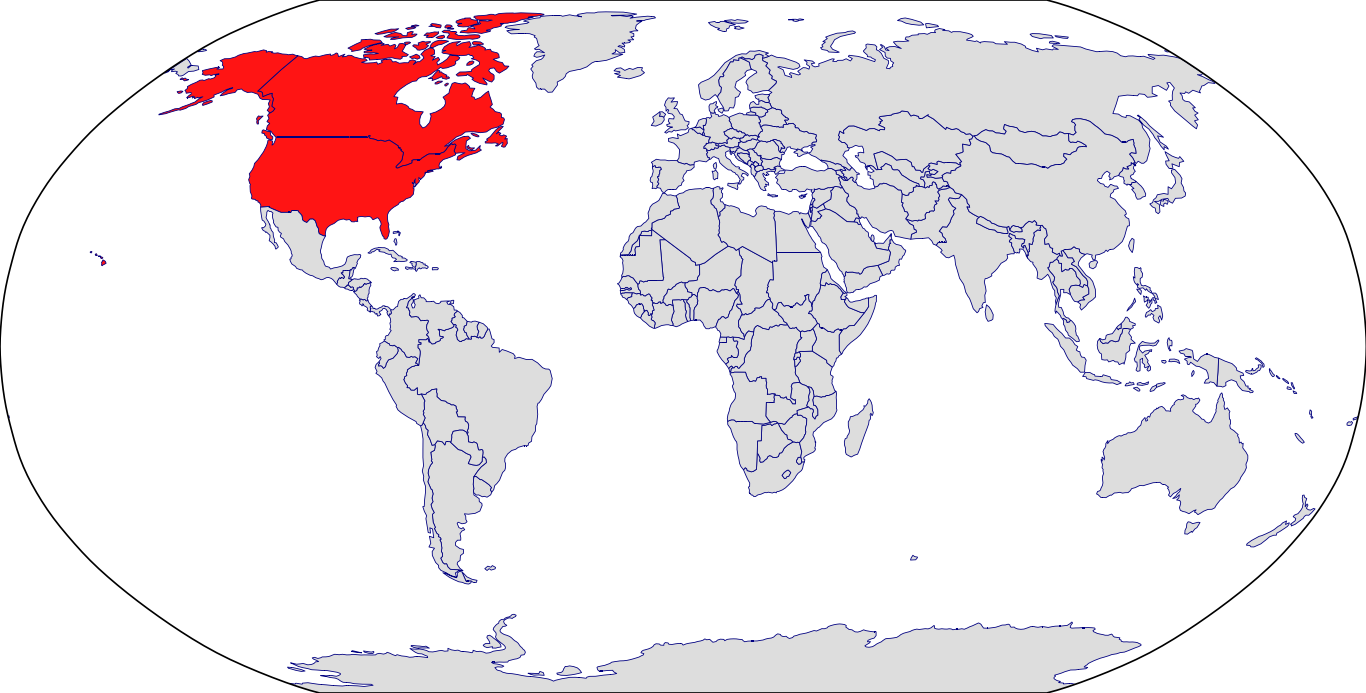
Countries in Clinic: Canada, United States
Active Clinical Trial Count: 1
Recent & Upcoming Milestones
Highest Development Phases
Phase 1: Colorectal Cancer
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT05948826 |
TORL3600-001 | P1 |
Active, not recruiting |
Colorectal Cancer |
2026-06-25 |
50% |
2025-10-11 |


