Product Description
Teriflunomide is in a class of medications called immunomodulatory agents. It is thought to work by decreasing inflammation and decreasing the action of immune cells that may cause nerve damage. (Sourced from: https://medlineplus.gov/druginfo/meds/a613010.html)
Mechanisms of Action: DHODH Inhibitor
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Oral
FDA Designation: *
Approval Status: Approved
Approved Countries: Argentina | Australia | Austria | Belgium | Bosnia | Brazil | Bulgaria | Canada | Chile | Colombia | Croatia | Cyprus | Czech | Denmark | Dominican Republic | Ecuador | Egypt | Estonia | European Medicines Agency | Finland | France | Germany | Greece | Hong Kong | Hungary | Iceland | India | Ireland | Israel | Italy | Korea | Latvia | Lebanon | Lithuania | Luxembourg | Malaysia | Malta | Mexico | Netherlands | New Zealand | Norway | Peru | Philippines | Poland | Portugal | Romania | Russia | Saudi Arabia | Serbia | Singapore | Slovakia | Slovenia | South Africa | Spain | Sweden | Switzerland | Taiwan | Tunisia | Turkey | Ukraine | United Arab Emirates | United Kingdom | United States | Uruguay
Approved Indications: None
Known Adverse Events: None
Company: Sanofi
Company Location: Europe
Company Founding Year: 1973
Additional Commercial Interests: None
Clinical Description
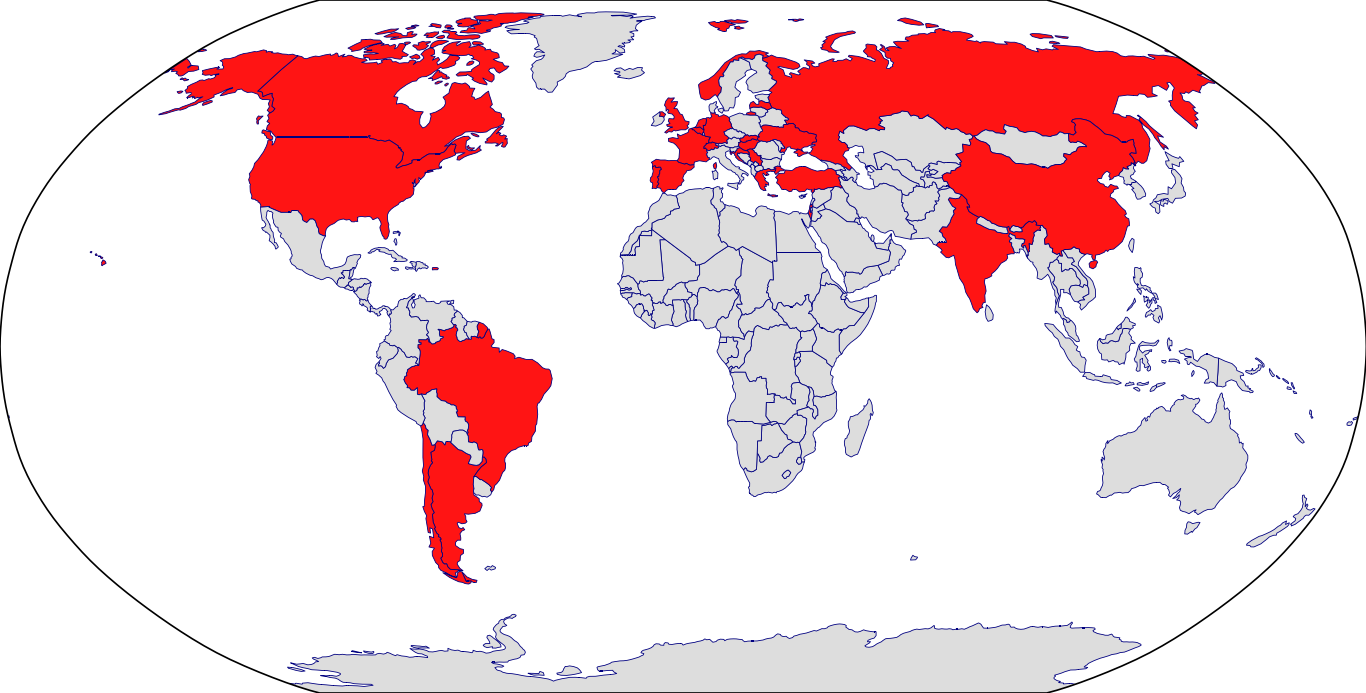
Countries in Clinic: Argentina, Belgium, Brazil, Canada, Chile, China, Croatia, Czech Republic, France, Germany, Greece, Hungary, India, Israel, Korea, Latvia, Netherlands, Norway, Portugal, Puerto Rico, Russia, Serbia, Slovakia, Spain, Switzerland, Turkey, Ukraine, United Kingdom, United States
Active Clinical Trial Count: 3
Recent & Upcoming Milestones
Highest Development Phases
Phase 3: Multiple Sclerosis
Phase 1: Multiple Sclerosis, Relapsing-Remitting|Multiple Sclerosis, Secondary Progressive
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
CTR20231591 |
CTR20231591 | P1 |
Completed |
Multiple Sclerosis, Secondary Progressive|Multiple Sclerosis, Relapsing-Remitting |
2023-08-01 |
2025-04-29 |
Patient Enrollment|Primary Completion Date|Start Date|Study Completion Date|Treatments|Trial Status |
|
NCT04410991 |
GEMINI 2 | P3 |
Completed |
Multiple Sclerosis |
2024-07-16 |
48% |
2024-09-20 |
|
CTR20222095 |
CTR20222095 | P1 |
Recruiting |
Multiple Sclerosis, Relapsing-Remitting|Multiple Sclerosis, Secondary Progressive |
None |
2025-04-29 |
Patient Enrollment|Treatments |


