Product Description
Mechanisms of Action: SRC Inhibitor
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Oral
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: AstraZeneca
Company Location: Europe
Company Founding Year: 1999
Additional Commercial Interests: None
Clinical Description
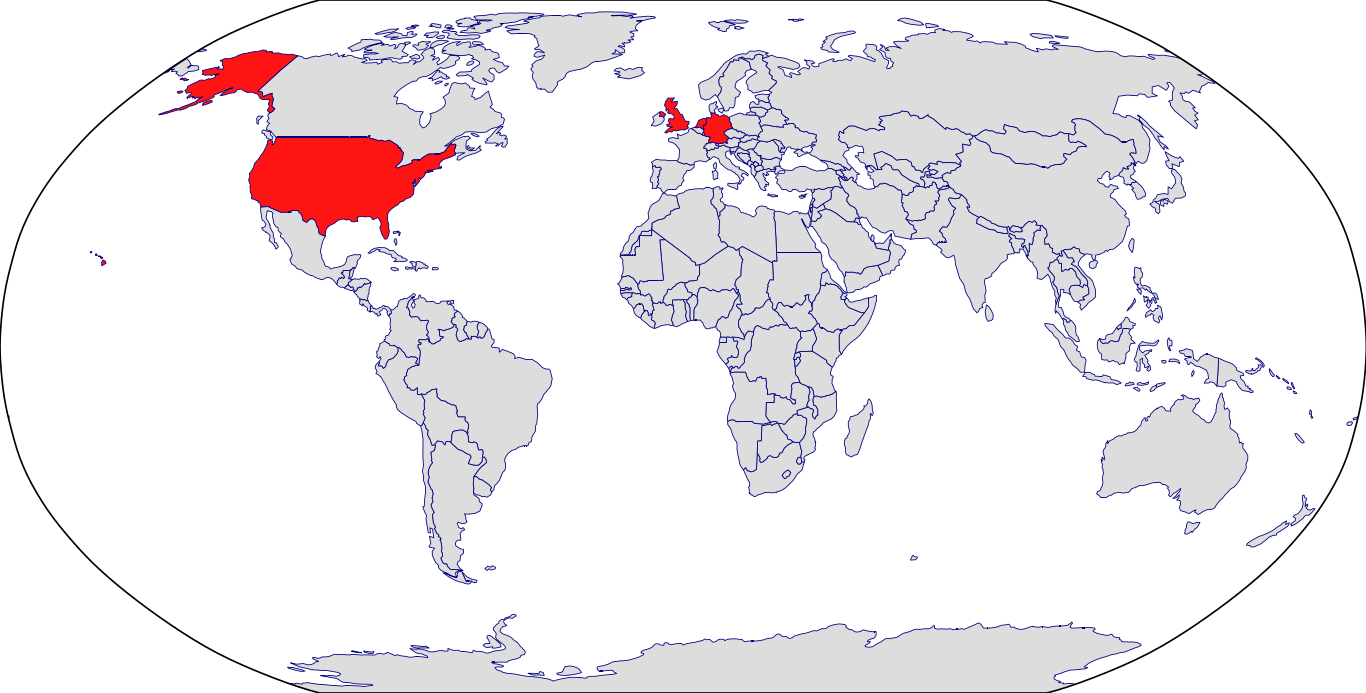
Countries in Clinic: Germany, Netherlands, United Kingdom, United States
Active Clinical Trial Count: 2
Recent & Upcoming Milestones
Highest Development Phases
Phase 2: Fibrodysplasia Ossificans Progressiva|Idiopathic Pulmonary Fibrosis|Myositis Ossificans
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT04307953 |
STOPFOP | P2 |
Recruiting |
Myositis Ossificans|Fibrodysplasia Ossificans Progressiva |
2025-05-06 |
12% |
2024-05-04 |
|
NCT04598919 |
STOP-IPF | P2 |
Active, not recruiting |
Idiopathic Pulmonary Fibrosis |
2024-09-15 |
24% |
2024-04-27 |
Primary Endpoints |


