Product Description
Prednisone is a synthetic, anti-inflammatory glucocorticoid that derives from cortisone. It is biologically inert and converted to prednisolone in the liver. Prednisone is an FDA-approved, delayed-release corticosteroid indicated as an anti-inflammatory or immunosuppressive agent to treat a broad range of diseases, including immunosuppressive/endocrine, rheumatic, collagen, dermatologic, allergic states, ophthalmic, respiratory, hematologic, neoplastic, edematous, gastrointestinal, acute exacerbations of multiple sclerosis, and as an anti-inflammatory and an antineoplastic agent. (Sourced from: https://pubmed.ncbi.nlm.nih.gov/30521230/)
Mechanisms of Action: GR Agonist, Immunosuppressive
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Oral
FDA Designation: *
Approval Status: Approved
Approved Countries: Algeria | Argentina | Australia | Austria | Bangladesh | Belgium | Bosnia | Brazil | Bulgaria | Canada | Chile | China | Colombia | Croatia | Cyprus | Czech | Denmark | Dominican Republic | Ecuador | Egypt | Finland | France | Germany | Hong Kong | Hungary | India | Indonesia | Ireland | Israel | Italy | Jordan | Korea | Lebanon | Lithuania | Malta | Mexico | Morocco | Netherlands | New Zealand | Pakistan | Peru | Philippines | Poland | Portugal | Romania | Russia | Saudi Arabia | Serbia | Singapore | Slovakia | Slovenia | South Africa | Spain | Sweden | Switzerland | Taiwan | Tunisia | Turkey | Ukraine | United Arab Emirates | United Kingdom | United States | Uruguay | Venezuela | Vietnam
Approved Indications: None
Known Adverse Events: None
Company: Johnson & Johnson
Company Location: Eastern America
Company Founding Year: 1886
Additional Commercial Interests: None
Clinical Description
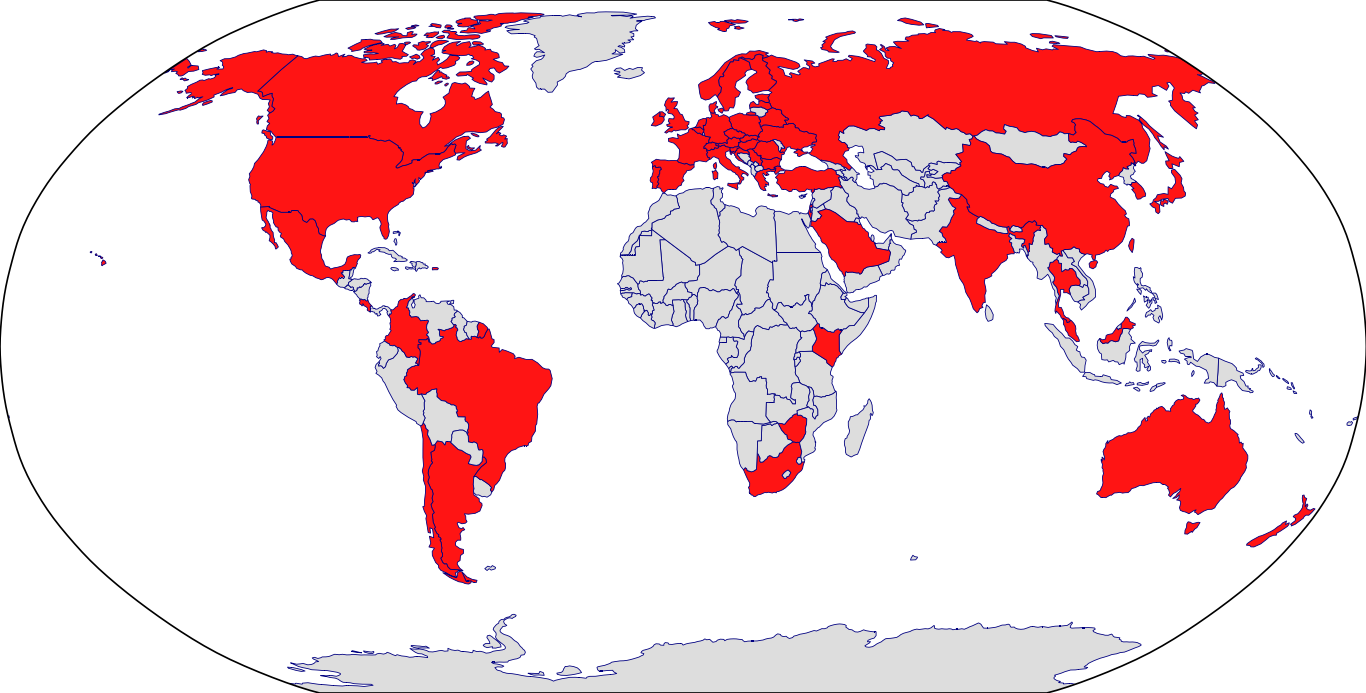
Countries in Clinic: Argentina, Australia, Austria, Belarus, Belgium, Brazil, Bulgaria, Canada, Chile, China, Colombia, Croatia, Czech Republic, Denmark, Finland, France, Germany, Greece, Hong Kong, Hungary, India, Ireland, Israel, Italy, Japan, Korea, Latvia, Malaysia, Mexico, Netherlands, New Zealand, Norway, Poland, Portugal, Puerto Rico, Romania, Russia, Saudi Arabia, Serbia, Singapore, Slovakia, South Africa, South Korea, Spain, Sweden, Switzerland, Taiwan, Thailand, Turkey, Ukraine, United Kingdom, United States, Unknown Location
Active Clinical Trial Count: 66
Recent & Upcoming Milestones
Highest Development Phases
Phase 3: Acute Lymphoid Leukemia|Asthma, Allergic|Bronchiolitis Obliterans|Conjunctivitis, Allergic|Diffuse Large B-Cell Lymphoma|Drug Hypersensitivity|Erythema Multiforme|Giant Cell Arteritis|Graft vs Host Disease|Histiocytosis|Hodgkin Lymphoma|Kidney Diseases|Kidney Transplant|Lymphoma|Lymphoma, B-Cell|Multiple Myeloma|Other|Pemphigoid, Bullous|Polymyalgia Rheumatica|Precursor Cell Lymphoblastic Leukemia-Lymphoma|Prostate Cancer|Pyoderma Gangrenosum|Rhinitis, Allergic|T-Cell Peripheral Lymphoma
Phase 2: Adenocarcinoma|Allogeneic Stem Cell Transplant|Burkitt Lymphoma|COVID-19|Cardiomyopathy, Dilated|Depressive Disorder, Major|Fetal Diseases|Follicular Lymphoma|GM1 Gangliosidosis|Gangliosidoses|Gangliosidosis, GM1|Heart Failure|Lung Cancer|Lymphoma, Non-Hodgkin|Lymphoproliferative Disorders|Precursor T-Cell Lymphoblastic Leukemia-Lymphoma|Pregnancy Complications, Infectious|Pregnancy Outcomes|Radiation Pneumonitis|T-Cell Leukemia|T-Cell Lymphoma
Phase 1: Acute Myeloid Leukemia|B-Cell Leukemia|Blast Crisis|Chronic Lymphoid Leukemia|Chronic Myeloid Leukemia|Myelogenous, Chronic, BCR-ABL Positive Leukemia
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
2024-516905-22-00 |
2024-516905-22-00 | P3 |
Active, not recruiting |
Multiple Myeloma |
2031-12-31 |
2025-05-02 |
Treatments |
|
NCT06072131 |
CRESCENDO | P3 |
Recruiting |
T-Cell Peripheral Lymphoma |
2030-07-01 |
18% |
2024-11-27 |
|
NCT07097363 |
RG1125011 | P2 |
Recruiting |
T-Cell Lymphoma|Lymphoma, Non-Hodgkin|Diffuse Large B-Cell Lymphoma|Burkitt Lymphoma |
2030-05-31 |
12% |
2025-12-18 |
Primary Endpoints|Start Date|Treatments|Trial Status |
NCT07412470 |
FREXERA | P3 |
Not yet recruiting |
Kidney Transplant |
2029-08-15 |
33% |
2026-02-18 |
|
NCT05848765 |
REFRACT | P2 |
Recruiting |
Follicular Lymphoma |
2029-05-31 |
78% |
2024-11-27 |
|
NCT04175431 |
RG1004972 | P2 |
Recruiting |
Adenocarcinoma|Prostate Cancer |
2028-07-01 |
12% |
2025-12-19 |
Primary Completion Date|Primary Endpoints|Study Completion Date |
NCT06738368 |
RG1124788 | P2 |
Recruiting |
Precursor Cell Lymphoblastic Leukemia-Lymphoma|T-Cell Leukemia|Acute Lymphoid Leukemia|Precursor T-Cell Lymphoblastic Leukemia-Lymphoma|Burkitt Lymphoma |
2028-01-31 |
12% |
2026-01-31 |
|
NCT03952637 |
19-HG-0101 | P2 |
Recruiting |
Gangliosidoses|GM1 Gangliosidosis|Gangliosidosis, GM1 |
2028-01-01 |
12% |
2025-08-27 |
Primary Endpoints |
NCT06585774 |
INCA34176-357 | P3 |
Recruiting |
Graft vs Host Disease|Bronchiolitis Obliterans |
2027-09-28 |
35% |
2024-12-06 |
Primary Endpoints|Treatments|Trial Status |
NCT06954805 |
AAAV2404 | P2 |
Recruiting |
Lymphoma, B-Cell|Lymphoproliferative Disorders |
2027-04-14 |
2025-05-03 |
Primary Endpoints|Treatments |
|
NCT06624670 |
U1111-1306-8055 | P3 |
Recruiting |
Pyoderma Gangrenosum |
2027-04-12 |
24% |
2025-09-20 |
Primary Completion Date|Primary Endpoints|Study Completion Date |
jRCT2033240447 |
jRCT2033240447 | P2 |
Recruiting |
Cardiomyopathy, Dilated|Heart Failure |
2027-03-31 |
|||
NCT03595917 |
NCT03595917 | P1 |
Recruiting |
Acute Myeloid Leukemia|Chronic Myeloid Leukemia|Blast Crisis|Acute Lymphoid Leukemia|Chronic Lymphoid Leukemia|B-Cell Leukemia|Lymphoma, B-Cell|Burkitt Lymphoma|Myelogenous, Chronic, BCR-ABL Positive Leukemia |
2026-11-01 |
12% |
2025-11-05 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments |
NCT03117751 |
TOT17 | P3 |
Active, not recruiting |
Acute Lymphoid Leukemia|Precursor Cell Lymphoblastic Leukemia-Lymphoma |
2026-09-30 |
10% |
2024-11-27 |
Primary Endpoints|Treatments |
NCT02684708 |
EuroNet-PHL-C2 | P3 |
Active, not recruiting |
Hodgkin Lymphoma |
2026-09-30 |
2021-05-14 |
Primary Endpoints|Treatments|Trial Status |
|
2024-511383-88-00 |
SC-3C2A | P3 |
Active, not recruiting |
Asthma, Allergic|Rhinitis, Allergic|Conjunctivitis, Allergic |
2026-09-30 |
2025-05-02 |
Treatments |
|
NCT04980222 |
GO43075 | P2 |
Active, not recruiting |
Lymphoma |
2026-09-30 |
12% |
2025-11-19 |
Primary Completion Date|Primary Endpoints|Treatments |
2023-508614-41-00 |
2023-0038 | P2 |
Recruiting |
Allogeneic Stem Cell Transplant |
2026-09-01 |
2025-05-02 |
Treatments |
|
2022-000712-59 |
SEM-CORTICO (Severe Erythema Multiforme - CORTICO) | P3 |
Active, not recruiting |
Erythema Multiforme |
2026-08-07 |
|||
NCT04812366 |
GUNS | P2 |
Recruiting |
Prostate Cancer |
2026-04-01 |
12% |
2022-02-08 |
Primary Endpoints|Treatments |
NCT06172361 |
ITTGPMR | P3 |
Recruiting |
Polymyalgia Rheumatica|Giant Cell Arteritis |
2025-09-30 |
2024-07-30 |
Primary Endpoints|Start Date|Treatments|Trial Status |
|
NCT05267600 |
BALLAD | P3 |
Completed |
Pemphigoid, Bullous |
2024-09-13 |
57% |
2024-10-16 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Trial Status |
NCT02496585 |
NCT02496585 | P2 |
Completed |
Radiation Pneumonitis|Lung Cancer |
2024-04-12 |
24% |
2025-08-27 |
Primary Endpoints|Treatments |
NCT05288166 |
CYCLONE 3 | P3 |
Active, not recruiting |
Drug Hypersensitivity|Prostate Cancer |
2024-02-15 |
17% |
2025-06-28 |
|
2017-000390-37 |
MASTER-ANCA | P3 |
Active, not recruiting |
Kidney Diseases |
2023-12-01 |
2022-03-13 |
Treatments |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
02/16/2026 |
News Article |
Lilly's Retevmo (selpercatinib) delivers substantial event-free survival benefit as an adjuvant therapy in early-stage RET fusion-positive lung cancer |
|
02/12/2026 |
News Article |
Merck Advances Treatment of Bladder and Kidney Cancers with New Data at 2026 ASCO GU Cancers Symposium |
|
02/10/2026 |
News Article |
Incyte Reports Fourth Quarter and Full Year 2025 Financial Results |
|
01/29/2026 |
News Article |
AB Science receives notice of allowance for US patent covering masitinib in the treatment of metastatic castrate resistant prostate cancer |


