Product Description
Lumefantrine is an antimalarial agent used to treat acute uncomplicated malaria. It is administered in combination with artemether for improved efficacy. (Sourced from: https://pubchem.ncbi.nlm.nih.gov/compound/Lumefantrine)
Mechanisms of Action: Hematin Inhibitor, Nucleic Acid Synthesis Inhibitor, Protein Synthesis Inhibitor
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Oral
FDA Designation: *
Approval Status: Not Approved
Approved Countries: Australia | Austria | Bangladesh | Belgium | Brazil | Bulgaria | Chile | China | Ecuador | Egypt | France | Greece | India | Indonesia | Ireland | Japan | Malaysia | Netherlands | New Zealand | Pakistan | Peru | Portugal | Slovenia | South Africa | Spain | Sweden | Switzerland | Thailand | Ukraine | United Kingdom | United States | Venezuela | Vietnam
Approved Indications: None
Known Adverse Events: None
Company: Novartis
Company Location: Europe
Company Founding Year: 1996
Additional Commercial Interests: None
Clinical Description
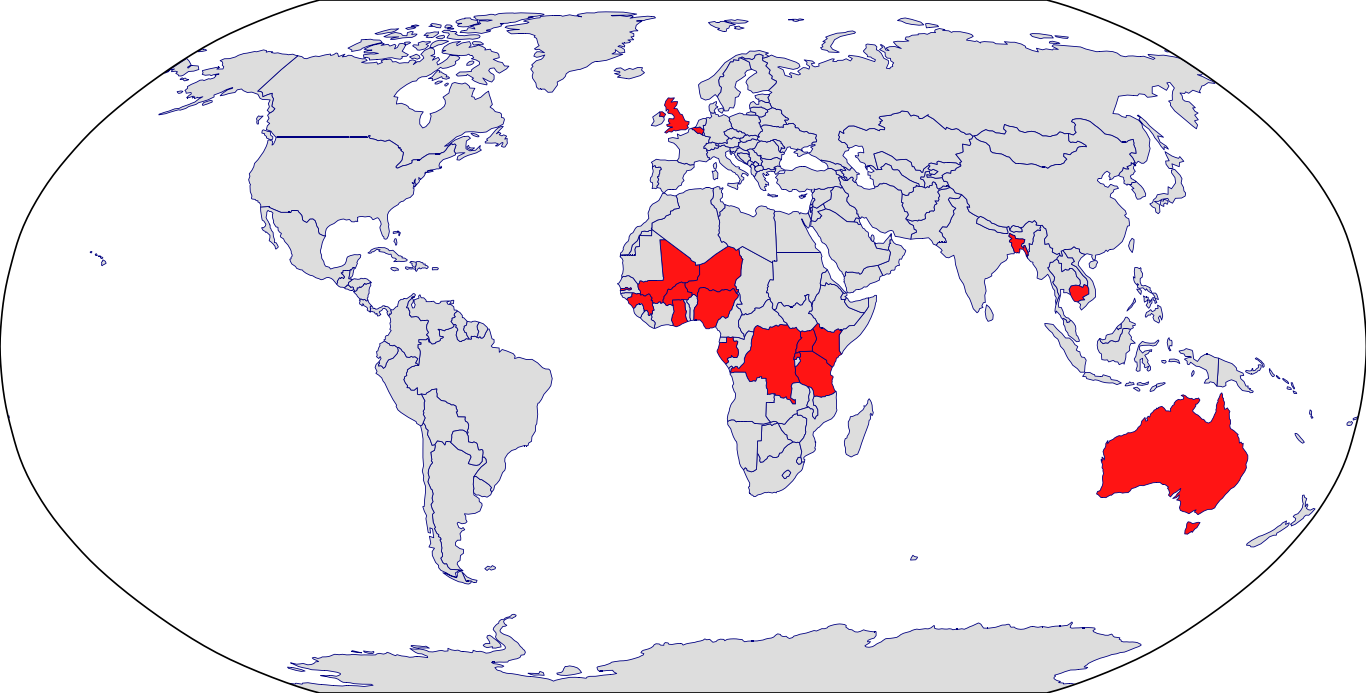
Countries in Clinic: Bangladesh, Burkina Faso, Cambodia, Congo, Gabon, Ghana, Guinea, Kenya, Mali, Niger, Nigeria, Rwanda, Tanzania, Uganda, Unknown Location
Active Clinical Trial Count: 4
Recent & Upcoming Milestones
Highest Development Phases
Phase 3: Malaria, Falciparum
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT07235033 |
PLATINUM | P2 |
Completed |
Malaria, Falciparum |
2025-03-05 |
2025-11-20 |
Primary Endpoints|Treatments |
|
NCT04546633 |
KALUMI | P2 |
Completed |
Malaria, Falciparum |
2024-08-13 |
12% |
2024-09-26 |
Patient Enrollment|Primary Endpoints|Treatments |
NCT03923725 |
DeTACT-Africa | P3 |
Completed |
Malaria, Falciparum |
2024-03-15 |
2025-11-19 |
Patient Enrollment|Primary Endpoints|Study Completion Date|Treatments|Trial Status |
|
NCT03939104 |
DeTACT-ASIA | P3 |
Completed |
Malaria, Falciparum |
2023-01-06 |
2024-03-21 |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
11/12/2025 |
News Article |
Novartis Phase III trial for next-generation malaria treatment KLU156 (GanLum) meets primary endpoint, with potential to combat antimalarial resistance |
|
07/22/2025 |
News Article |
Hesperos Demonstrates First Digital Twin of Human Disease Using Organ-on-a-Chip Platform |
|
07/17/2025 |
News Article |
Novartis reports strong Q2 with double-digit sales growth and core margin expansion; raises FY 2025 core operating income guidance |
|
07/08/2025 |
News Article |
Novartis receives approval for first malaria medicine for newborn babies and young infants |


