Product Description
KER-012 is designed to bind to and inhibit the signaling of TGF-ß ligands, including activin A and activin B, which are key regulators of bone remodeling that act to suppress bone growth. (Sourced from: https://ir.kerostx.com/news-releases/news-release-details/keros-therapeutics-presents-results-preclinical-study-ker-012)
Mechanisms of Action: Activin A Inhibitor, Activin B Inhibitor
Novel Mechanism: Yes
Modality: Peptide/Protein
Route of Administration: Subcutaneous
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: Keros Therapeutics
Company Location:
Company Founding Year: None
Additional Commercial Interests: None
Clinical Description
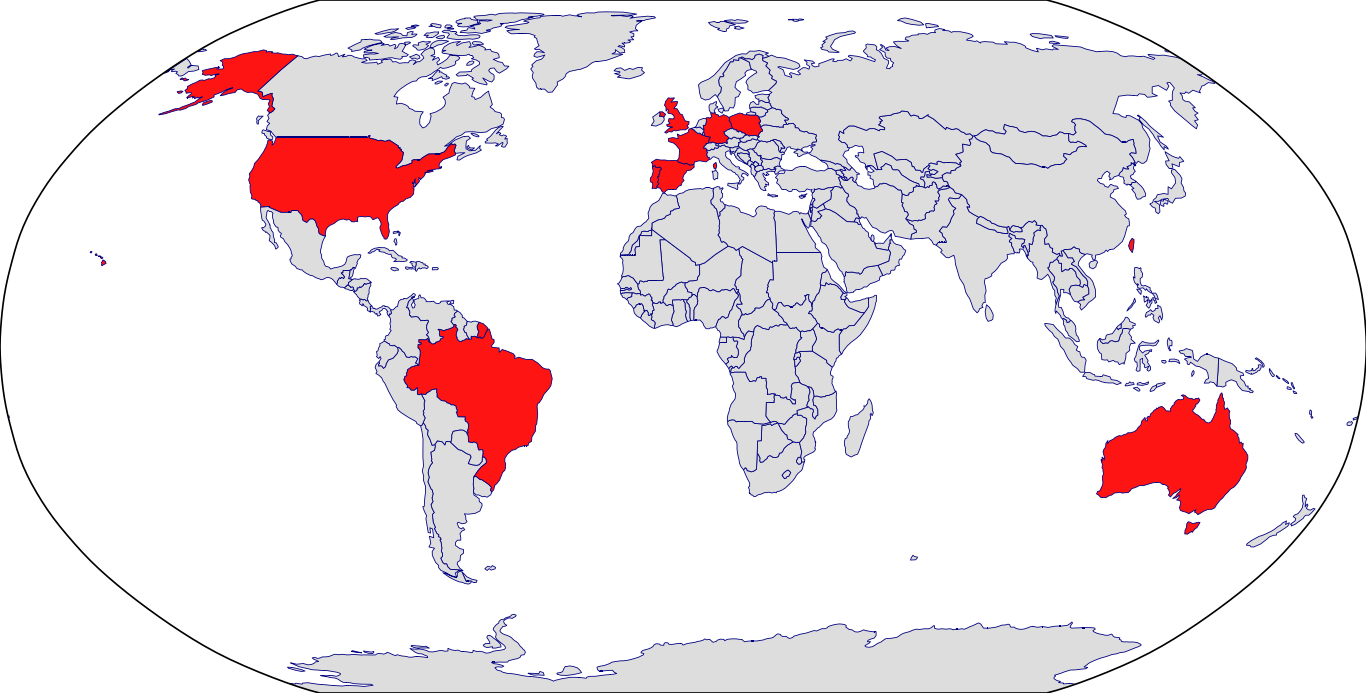
Countries in Clinic: Australia, Brazil, France, Germany, Korea, Poland, Portugal, Spain, Taiwan, United Kingdom, United States
Active Clinical Trial Count: 2
Recent & Upcoming Milestones
- Clinical Outcomes Reported - Keros Therapeutics presented P2 Hypertension|Hypertension, Pulmonary results on 2024-02-02 for Cibotercept
Highest Development Phases
Phase 2: Familial Primary Pulmonary Hypertension|Hypertension, Pulmonary
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT05975905 |
TROPOS Study | P2 |
Completed |
Hypertension, Pulmonary|Familial Primary Pulmonary Hypertension |
2025-03-05 |
12% |
2025-05-08 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments|Trial Status |
2022-502378-17-00 |
KER-012-A201 | P2 |
Completed |
Hypertension, Pulmonary |
2025-01-14 |
2025-05-02 |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
06/09/2025 |
News Article |
ADAR1 Issues Statement on Keros Therapeutics' Troubling 2025 Director Election Results and Insufficient Capital Return Proposal |
|
05/29/2025 |
News Article |
Keros Therapeutics Announces TROPOS Topline Data and Corporate Restructuring |
|
05/27/2025 |
News Article |
Leading Independent Proxy Advisory Firm Glass Lewis Recommends Stockholders Vote "FOR" All of Keros' Director Nominees |
|
05/19/2025 |
News Article |
Keros Therapeutics Highlights Commitment to Maximizing Stockholder Value |


