Product Description
autologous adipose tissue derived mesenchymal stem cells (AdMSC) (Sourced from: https://clinicaltrials.gov/ct2/show/NCT02674399)
Mechanisms of Action: Stem Cell Therapy
Novel Mechanism: No
Modality: Cell Therapy
Route of Administration: Injection
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: R-Bio
Company Location: Europe
Company Founding Year: 1988
Additional Commercial Interests: None
Clinical Description
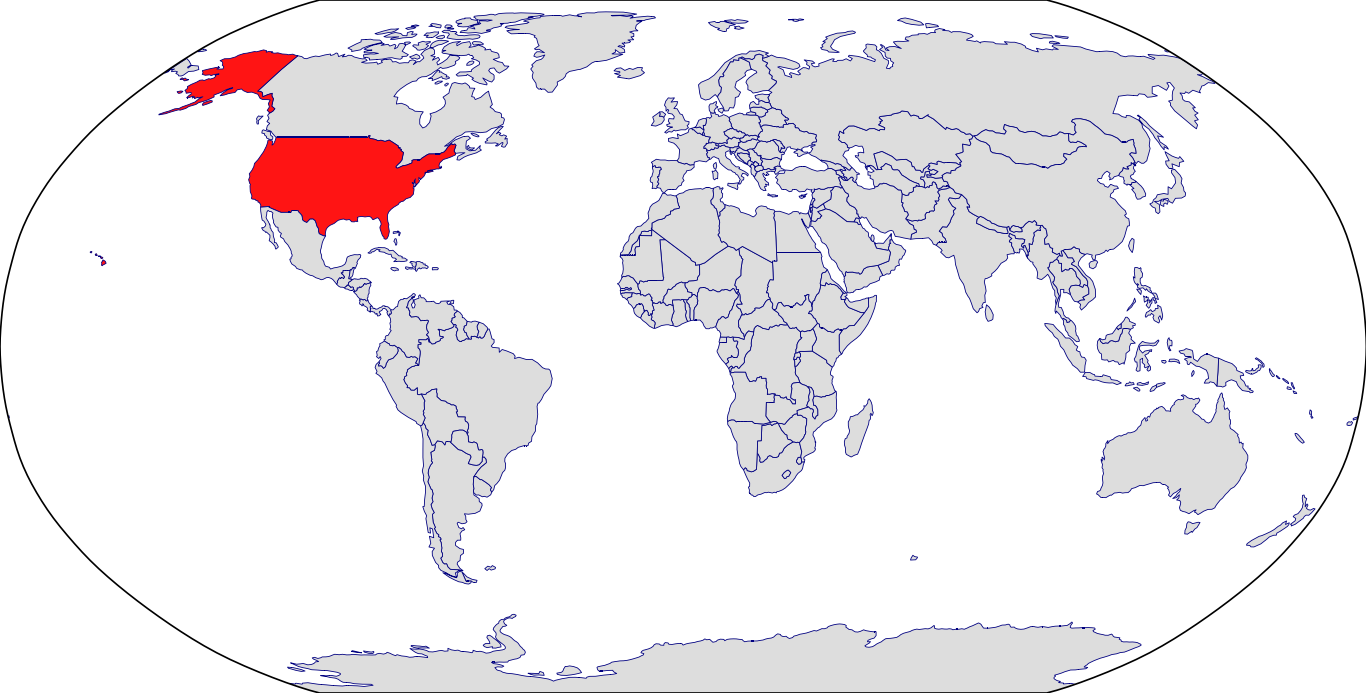
Countries in Clinic: Korea, United States
Active Clinical Trial Count: 2
Recent & Upcoming Milestones
Highest Development Phases
Phase 3: Osteoarthritis, Knee
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT04427930 |
BSR-CTph3-JS1_FU | P3 |
Active, not recruiting |
Osteoarthritis, Knee |
2026-12-23 |
20% |
2022-09-29 |
Patient Enrollment|Primary Endpoints|Treatments|Trial Status |
NCT04368806 |
JS-OAP3-US01 | P3 |
Recruiting |
Osteoarthritis, Knee |
2026-12-30 |
18% |
2025-07-25 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments|Trial Status |


