Product Description
J2H-1702 is a novel 11beta-HSD1 inhibitor, and the inhibition of 11beta-HSD1 has been shown to improve insulin sensitivity, reduce inflammation, and prevent the development of nonalcoholic steatohepatitis (NASH) in preclinical models. (Sourced from: https://pubmed.ncbi.nlm.nih.gov/37743843/)
Mechanisms of Action: 11b-HSD Inhibitor
Novel Mechanism: Yes
Modality: Small Molecule
Route of Administration: N/A
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: J2H Biotech
Company Location: Asia Pacific
Company Founding Year: None
Additional Commercial Interests: None
Clinical Description
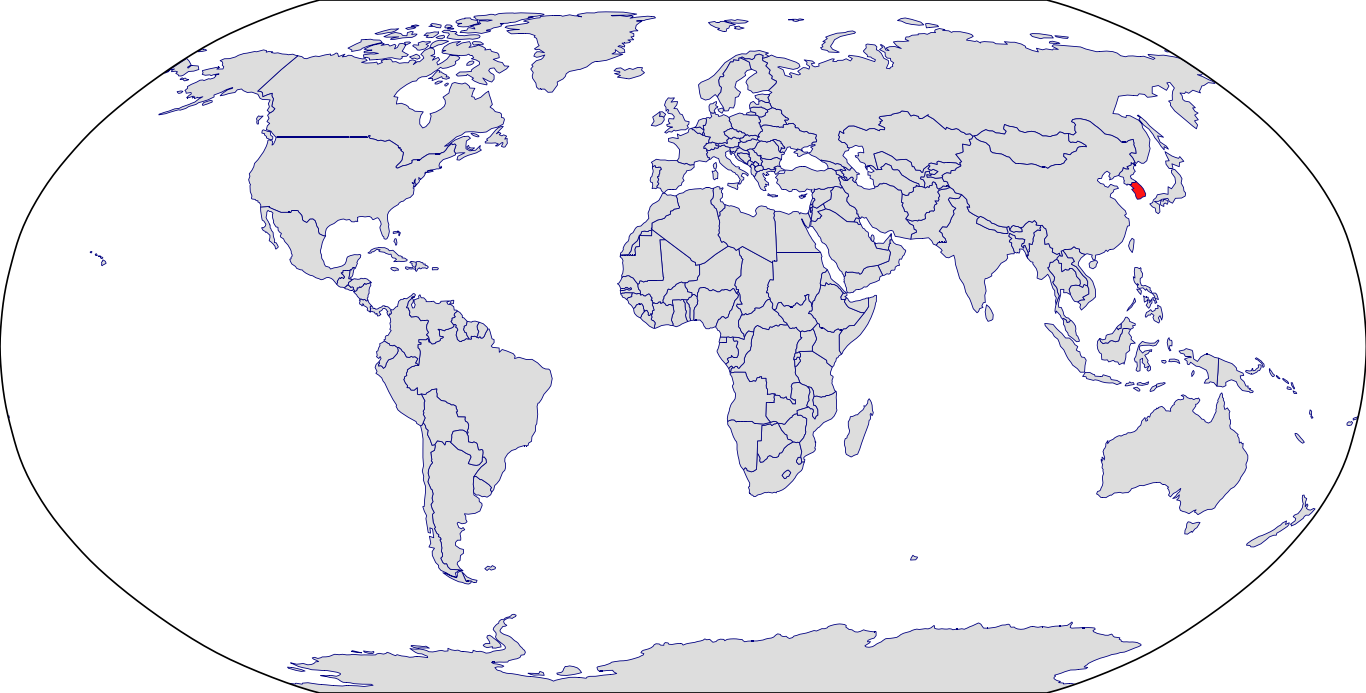
Countries in Clinic: South Korea
Active Clinical Trial Count: 1
Recent & Upcoming Milestones
Highest Development Phases
Phase 2: Fatty Liver, Alcoholic|Hepatitis, Alcoholic|Non-alcoholic Steatohepatitis
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT06297434 |
JH-221-201 | P2 |
Completed |
Fatty Liver, Alcoholic|Non-alcoholic Steatohepatitis|Hepatitis, Alcoholic |
2025-06-26 |
12% |
2025-12-18 |
Patient Enrollment|Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments|Trial Status |


