Product Description
Izokibep is a unique antibody mimetic and potent interleukin-17A (IL-17A) inhibitor designed to overcome the limitations of existing monoclonal antibodies.
Mechanisms of Action: IL17A Inhibitor
Novel Mechanism: No
Modality: Antibody
Route of Administration: Subcutaneous
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: ACELYRIN
Company Location:
Company Founding Year: 2020
Additional Commercial Interests: Affibody
Clinical Description
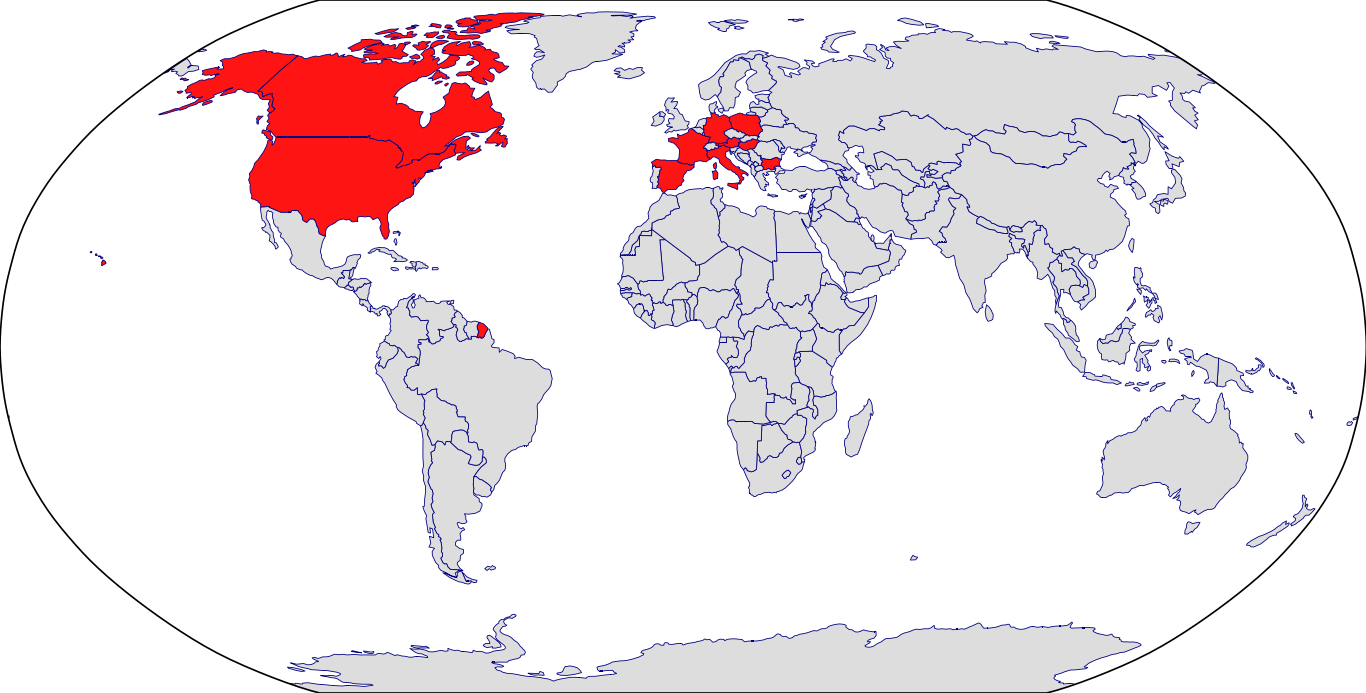
Countries in Clinic: Austria, Bulgaria, Canada, Czech Republic, France, Germany, Hungary, Italy, Poland, Spain, United States, Unknown Location
Active Clinical Trial Count: 6
Recent & Upcoming Milestones
- Clinical Outcomes Reported - ACELYRIN presented P2 Uveitis results on 2024-12-10 for Izokibep
- Clinical Outcomes Reported - ACELYRIN presented P3 Hidradenitis Suppurativa results on 2024-09-25 for Izokibep
- Clinical Outcomes Reported - ACELYRIN presented P2 Arthritis, Psoriatic|Arthritis results on 2024-06-15 for Izokibep
Highest Development Phases
Phase 3: Arthritis, Psoriatic|Hidradenitis Suppurativa
Phase 2: Uveitis, Intermediate|Uveitis, Posterior
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
2024-514975-16-00 |
21103 | P2 |
Completed |
Uveitis, Intermediate|Uveitis, Posterior |
2025-01-07 |
12% |
2025-05-02 |
Treatments |
jRCT2031230501 |
jRCT2031230501 | P3 |
Not yet recruiting |
Hidradenitis Suppurativa |
2025-12-31 |
|||
2022-501362-22-00 |
22104 | P3 |
Completed |
Arthritis, Psoriatic |
2024-10-03 |
2025-05-02 |
Treatments |
|
2021-005713-13 |
2021-005713-13 | P2 |
Completed |
Hidradenitis Suppurativa |
2024-02-21 |
85% |
2025-05-06 |
Patient Enrollment|Primary Completion Date|Study Completion Date|Treatments|Trial Status |
NCT05355805 |
NCT05355805 | P2 |
Completed |
Hidradenitis Suppurativa |
2023-08-02 |
85% |
2024-09-26 |
|
2022-503160-33-00 |
22107 | P3 |
Completed |
Hidradenitis Suppurativa |
2025-01-27 |
18% |
2025-05-02 |
Treatments |


