Product Description
Marinus is developing Ganaxolone as a treatment for seizure disorders. (Sourced from: https://marinuspharma.com/pipeline/science-pipeline/about-ganaxolone/)
Mechanisms of Action: GABA Modulator
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Oral
FDA Designation: Orphan Drug - Lennox Gastaut SyndromeOrphan Drug - Tuberous Sclerosis *
Approval Status: Approved
Approved Countries: United States
Approved Indications: None
Known Adverse Events: None
Company: Marinus
Company Location:
Company Founding Year: 2003
Additional Commercial Interests: None
Clinical Description
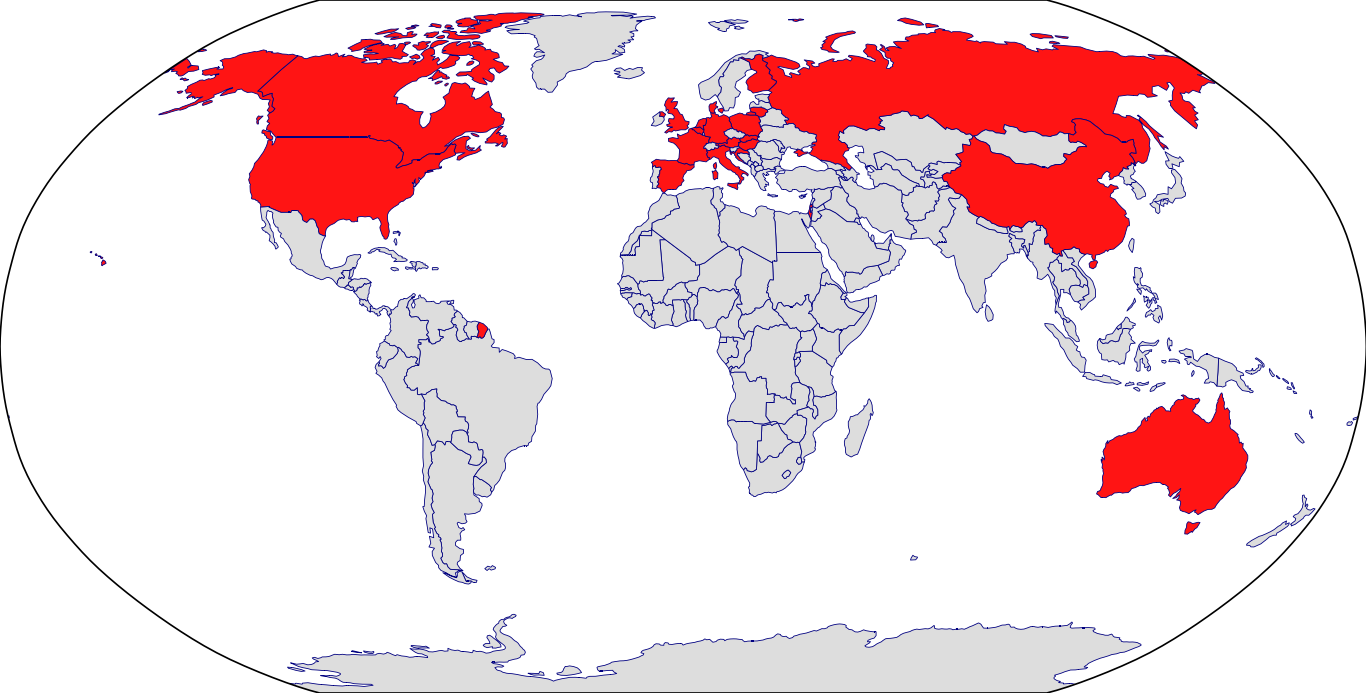
Countries in Clinic: Australia, Austria, Belgium, Canada, China, Croatia, Czech Republic, Denmark, Finland, France, Germany, Hungary, Israel, Italy, Lithuania, Poland, Slovakia, Spain, United Kingdom, United States
Active Clinical Trial Count: 6
Recent & Upcoming Milestones
- Clinical Outcomes Reported - Marinus presented P3 Tuberous Sclerosis results on 2024-10-24 for Ganaxolone
- Clinical Outcomes Reported - Marinus presented P3 Status Epilepticus results on 2024-10-17 for Ganaxolone
- Clinical Outcomes Reported - Marinus announced they will present P2 Tuberous Sclerosis results in 4Q24 for Ganaxolone
Highest Development Phases
Phase 3: CDKL5-deficiency Disorder|Epilepsy|Status Epilepticus|Tuberous Sclerosis
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT05249556 |
1042-CDD-3002 | P3 |
Not yet recruiting |
CDKL5-deficiency Disorder |
2028-09-01 |
16% |
2025-11-22 |
Primary Completion Date|Primary Endpoints|Start Date|Study Completion Date|Treatments |
NCT05323734 |
TrustTSC | P3 |
Completed |
Epilepsy|Tuberous Sclerosis |
2024-09-09 |
14% |
2025-07-12 |
Patient Enrollment|Primary Endpoints|Treatments |
NCT04391569 |
RAISE | P3 |
Completed |
Status Epilepticus |
2024-03-30 |
9% |
2025-05-31 |
Patient Enrollment|Primary Endpoints|Treatments |
2022-503067-15-00 |
1042-TSC-3002 | P3 |
Completed |
Epilepsy|Tuberous Sclerosis |
2024-11-25 |
2025-05-02 |
Treatments |
|
2021-003441-38 |
Adjunctive GNX Treatment Compared with Placebo in Children and Adults with TSC-related Epilepsy | P3 |
Completed |
Tuberous Sclerosis|Epilepsy |
2024-10-15 |
2025-05-06 |
Primary Completion Date|Study Completion Date|Treatments|Trial Status |
|
2022-502540-12-00 |
1042-SE-3004 | P3 |
Completed |
Status Epilepticus |
2024-04-23 |
2025-05-02 |
Treatments |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
08/11/2025 |
News Article |
The Galien Foundation Announces 2025 Prix Galien USA Nominees for "Best Biotechnology Product," "Best Pharmaceutical Product" and "Best Product for Rare/Orphan Diseases" |
|
06/25/2025 |
News Article |
Ovid Therapeutics Enters Agreement with Immedica Pharma AB for Sale of Future Ganaxolone Royalties |
|
12/30/2024 |
News Article |
Immedica to Acquire Biopharmaceutical Company Marinus Pharmaceuticals, Inc. |
|
11/12/2024 |
News Article |
Marinus Pharmaceuticals Provides Business Update and Reports Third Quarter 2024 Financial Results |


