Product Description
Mechanisms of Action: DDC Inhibitor, DR Agonist
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Oral
FDA Designation: *
Approval Status: Not Approved
Approved Countries: Australia | Austria | Belgium | Brazil | Bulgaria | Croatia | Cyprus | Czech | Denmark | Estonia | France | Germany | Hungary | India | Italy | Japan | Lithuania | New Zealand | Norway | Pakistan | Poland | Portugal | Russia | Slovakia | South Africa | Spain | Taiwan | Thailand | Turkey | United Arab Emirates | United Kingdom
Approved Indications: None
Known Adverse Events: None
Company: AbbVie
Company Location: Eastern America
Company Founding Year: 2013
Additional Commercial Interests: None
Clinical Description
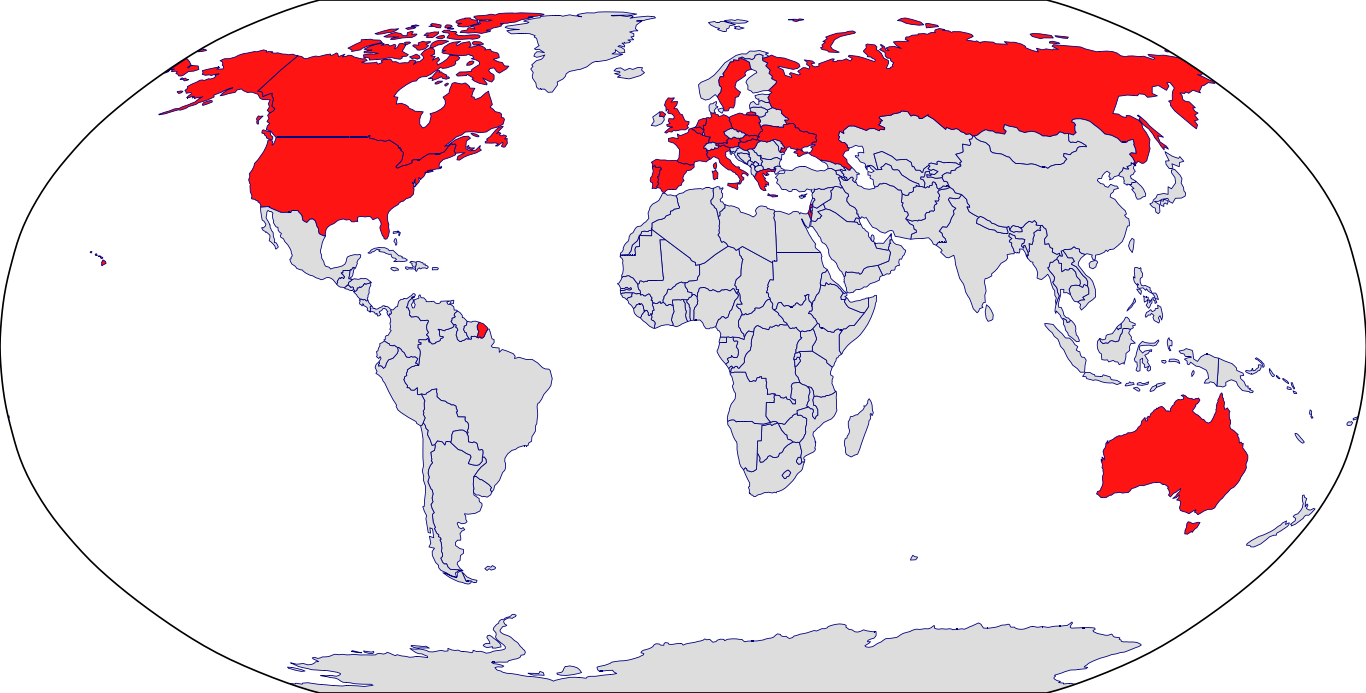
Countries in Clinic: Austria, Belgium, Czech Republic, France, Germany, Hungary, Israel, Italy, Netherlands, Poland, Portugal, Russia, Slovakia, Spain, Ukraine, United Kingdom, United States
Active Clinical Trial Count: 6
Recent & Upcoming Milestones
Highest Development Phases
Phase 3: Parkinson's Disease
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
2024-511940-20-00 |
ND0612-317 | P3 |
Active, not recruiting |
Parkinson's Disease |
2027-05-06 |
2025-05-02 |
Treatments |
|
2024-513548-27-00 |
ND0612H-012 | P2 |
Active, not recruiting |
Parkinson's Disease |
2027-05-23 |
2025-05-02 |
Treatments |
|
NCT02726386 |
BeyoND | P2 |
Active, not recruiting |
Parkinson's Disease |
2019-09-09 |
12% |
2024-01-20 |
Primary Completion Date|Primary Endpoints|Start Date|Treatments |
2018-004156-37 |
BouNDless | P3 |
Active, not recruiting |
Parkinson's Disease |
2023-05-18 |
2025-05-06 |
Treatments |
|
NCT04006210 |
BouNDless | P3 |
Active, not recruiting |
Parkinson's Disease |
2022-11-01 |
66% |
2023-11-08 |
Primary Endpoints |
2016-002033-30 |
iNDiGO | P3 |
Active, not recruiting |
Parkinson's Disease |
2017-04-27 |
2022-03-13 |
Treatments |


