Product Description
Defibrotide is approved for the treatment of adult and pediatric patients with hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), with renal or pulmonary dysfunction following hematopoietic stem cell transplantation (HSCT). (Sourced from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5135434/)
Mechanisms of Action: Unknown
Novel Mechanism: No
Modality: Peptide/Protein
Route of Administration: Intravenous
FDA Designation: *
Approval Status: Approved
Approved Countries: Australia | Austria | Belgium | Brazil | Canada | Croatia | Czech | Denmark | Estonia | European Medicines Agency | Finland | France | Germany | Greece | Hungary | Iceland | Ireland | Israel | Italy | Japan | Korea | Latvia | Lithuania | Netherlands | Norway | Pakistan | Poland | Portugal | Romania | Saudi Arabia | Slovakia | Slovenia | Sweden | Switzerland | United Kingdom | United States
Approved Indications: None
Known Adverse Events: None
Company: Jazz
Company Location: Europe
Company Founding Year: 2003
Additional Commercial Interests: None
Clinical Description
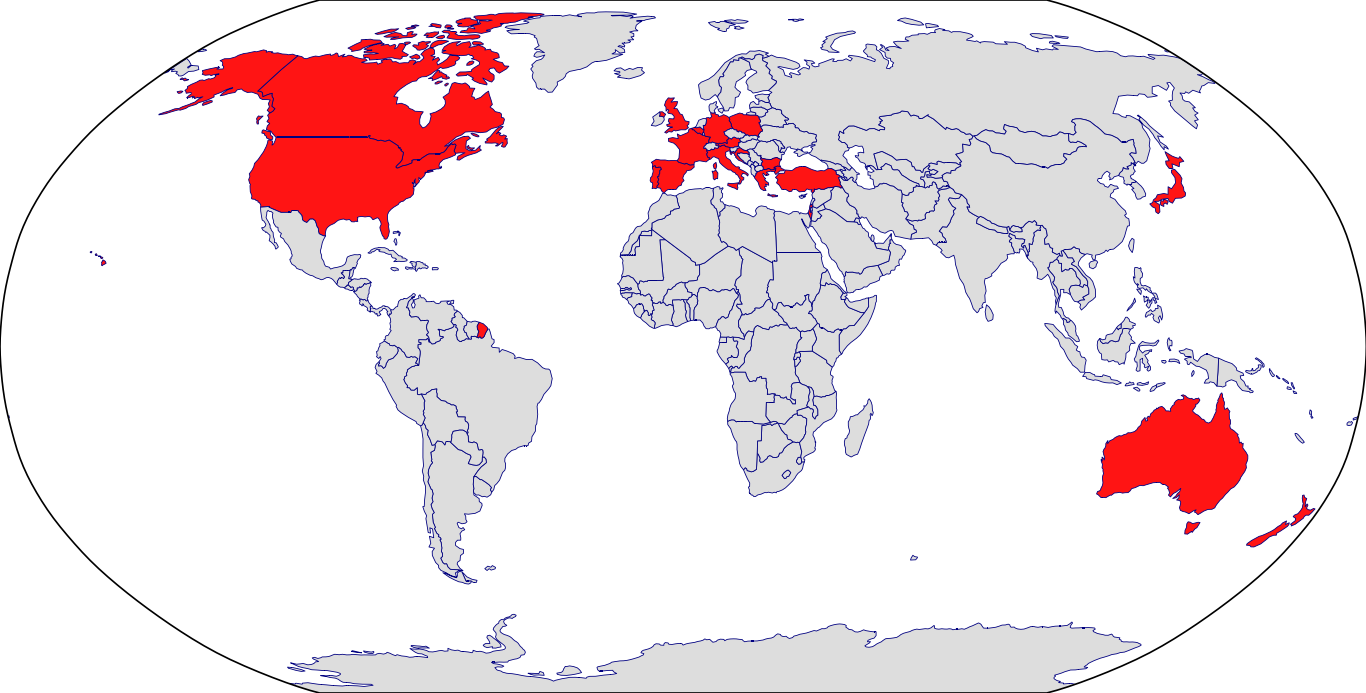
Countries in Clinic: United States
Active Clinical Trial Count: 2
Recent & Upcoming Milestones
- Clinical Outcomes Reported - Jazz presented P2 Anemia, Sickle Cell results on 2023-12-10 for Defibrotide
Highest Development Phases
Phase 2: COVID-19
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT04652115 |
2020P003203 | P2 |
Recruiting |
COVID-19 |
2024-09-01 |
14% |
2024-05-11 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments |
2020-001409-21 |
DEFACOVID | P2 |
Active, not recruiting |
COVID-19 |
2020-09-30 |
2022-03-13 |
Treatments |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
12/24/2025 |
News Article |
FDA Approves Omeros' YARTEMLEA® – First and Only Therapy Indicated for TA-TMA |
|
12/01/2025 |
News Article |
Circle Pharma Appoints Anne E. Borgman, M.D., as Chief Medical Officer |
|
11/13/2025 |
News Article |
Omeros Corporation Reports Third Quarter 2025 Financial Results |
|
02/20/2025 |
News Article |
Omeros Announces Robust Results for Narsoplimab Expanded Access Program in TA-TMA |


