Product Description
Dacomitinib is a multi-kinase receptor inhibitor used in the therapy of cases of non-small cell lung cancer that harbor activating mutations in the epidermal growth factor receptor gene (EGFR). Dacomitinib is associated with high rate of transient serum aminotransferase elevations during therapy but has not been linked to instances of clinically apparent acute liver injury. (Sourced from: https://pubchem.ncbi.nlm.nih.gov/compound/Dacomitinib)
Mechanisms of Action: TK Inhibitor, EGFR Inhibitor
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Oral
FDA Designation: *
Approval Status: Approved
Approved Countries: Argentina | Austria | Bangladesh | Belgium | Canada | Chile | Colombia | Croatia | Czech | Denmark | Ecuador | Egypt | Estonia | European Medicines Agency | Finland | Germany | Greece | Hong Kong | Hungary | Iceland | Indonesia | Ireland | Israel | Italy | Japan | Jordan | Korea | Latvia | Lebanon | Lithuania | Malaysia | Mexico | Netherlands | Norway | Peru | Philippines | Poland | Portugal | Romania | Russia | Saudi Arabia | Singapore | Slovakia | Slovenia | Spain | Sweden | Switzerland | Taiwan | Turkey | United Arab Emirates | United Kingdom | United States | Uruguay
Approved Indications: None
Known Adverse Events: None
Company: Pfizer
Company Location: Eastern America
Company Founding Year: 1849
Additional Commercial Interests: None
Clinical Description
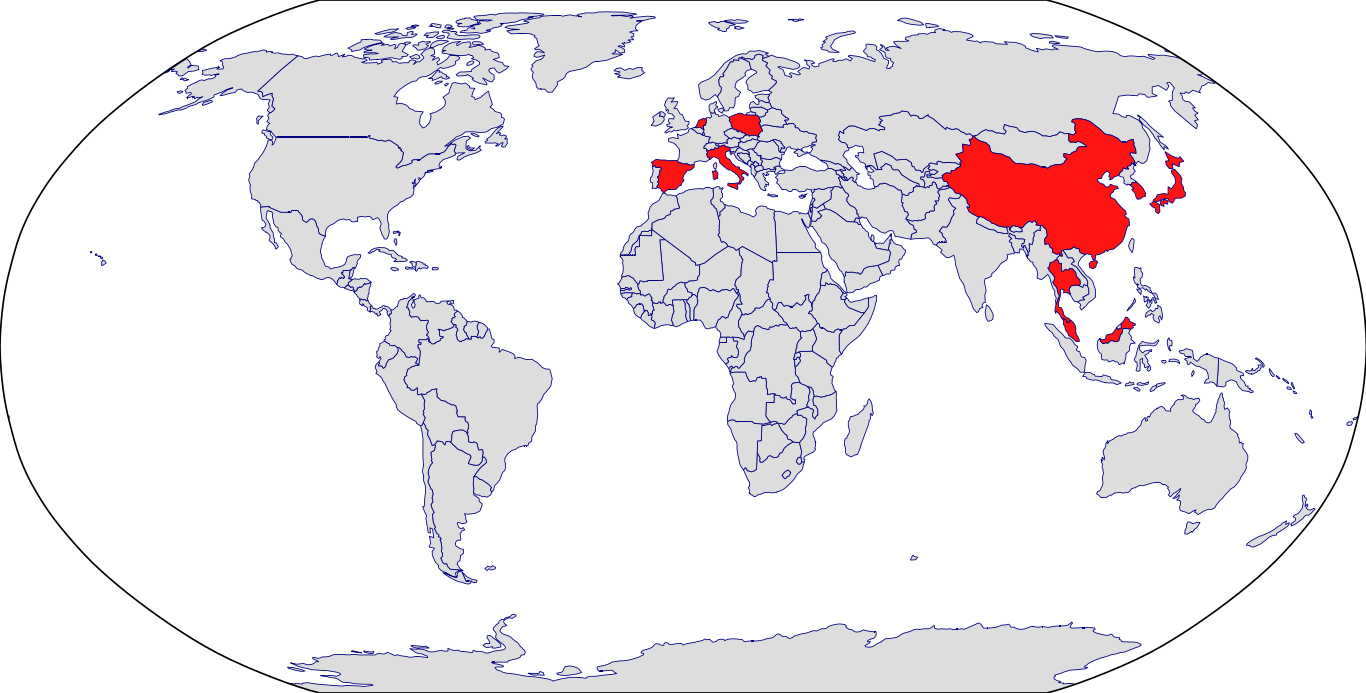
Countries in Clinic: Hong Kong, Korea, Malaysia, Netherlands, Singapore, Thailand
Active Clinical Trial Count: 2
Recent & Upcoming Milestones
Highest Development Phases
Phase 2: Abnormalities, Multiple|Hodgkin Lymphoma|Lymphoma, B-Cell|Lymphoma, Non-Hodgkin|Multiple Myeloma|Non-Small-Cell Lung Cancer|Vision, Low
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT02925234 |
DRUP | P2 |
Recruiting |
Lymphoma, Non-Hodgkin|Abnormalities, Multiple|Hodgkin Lymphoma|Lymphoma, B-Cell|Vision, Low|Multiple Myeloma |
2027-09-01 |
12% |
2024-01-25 |
Primary Endpoints |
NCT04027647 |
ATORG-003 | P2 |
Active, not recruiting |
Non-Small-Cell Lung Cancer |
2026-03-31 |
12% |
2024-06-26 |
Primary Endpoints|Treatments |


