Product Description
CID-103 is a fully human IgG1 anti-CD38 monoclonal antibody that recognizes a unique epitope on CD38. It was engineered to have strong activity against CD38 malignant cells and to reduce certain safety issues observed with existing treatments. Preclinical data of CID-103 demonstrates enhanced activity against a broad array of malignancies which express CD38 and demonstrates a better preclinical safety profile when compared to other CD38 mAbs. These attributes offer the potential for accelerated development and regulatory review, including rapid advancement into earlier lines of therapy. (Sourced from: https://www.casipharmaceuticals.com/product-pipeline/anti-cd38-monoclonal-antibody)
Mechanisms of Action: CD38 Inhibitor
Novel Mechanism: No
Modality: Antibody
Route of Administration: Intravenous
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: CASI
Company Location: Asia Pacific
Company CEO: Wei-Wu He
Additional Commercial Interests: None
Clinical Description
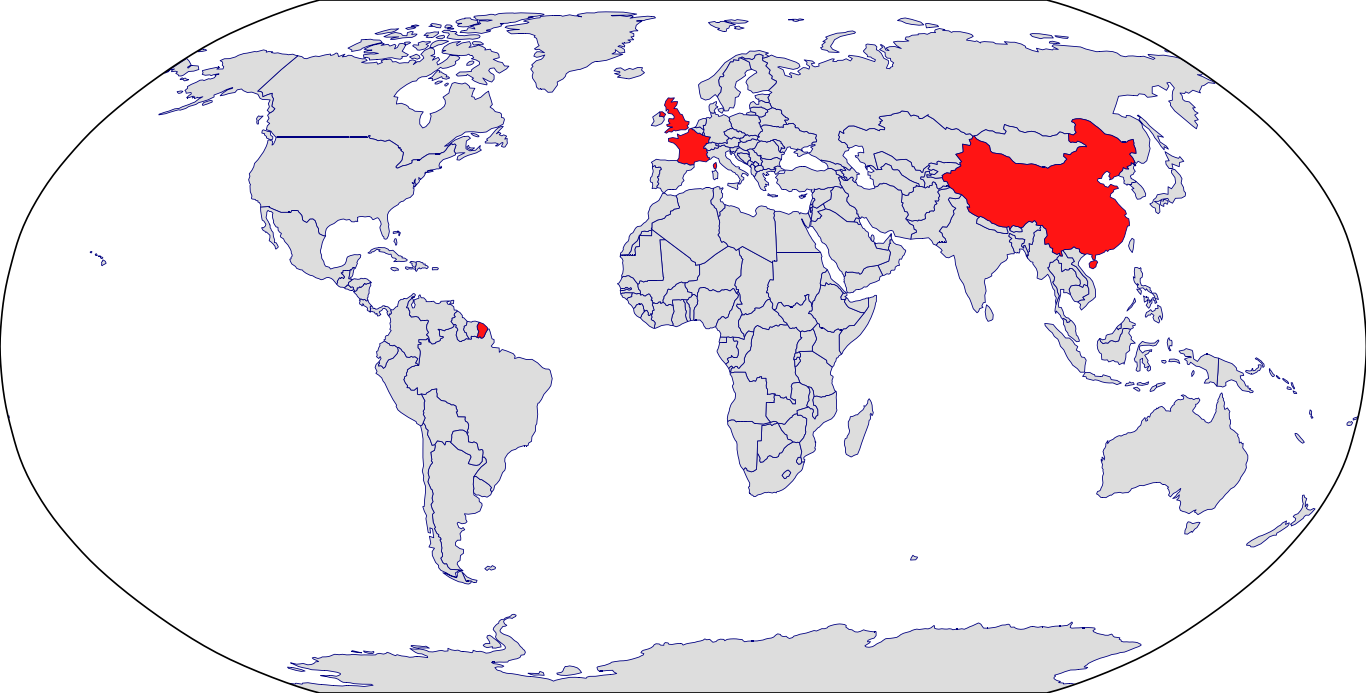
Countries in Clinic: China, France, United Kingdom
Active Clinical Trial Count: 2
Recent & Upcoming Milestones
- Clinical Outcomes Reported - CASI presented P1 Thrombocytopenia results on 2025-12-08 for CID-103
- Clinical Outcomes Reported - CASI presented P1 Thrombocytopenia results on 2025-12-07 for CID-103
Highest Development Phases
Phase 2: Purpura, Thrombocytopenic, Idiopathic|Thrombocytopenia
Phase 1: Multiple Myeloma
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT04758767 |
CASI-CID-103-101 | P1 |
Completed |
Multiple Myeloma |
2023-01-19 |
37% |
2025-04-04 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments|Trial Status |
NCT07017725 |
CASI-CID-103-201 | P2 |
Recruiting |
Thrombocytopenia|Purpura, Thrombocytopenic, Idiopathic |
2026-06-01 |
50% |
2025-06-13 |
Primary Endpoints|Treatments |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
12/23/2025 |
News Article |
CASI Pharmaceuticals Receives Extension from Nasdaq to Meet Listing Requirements |
|
12/11/2025 |
News Article |
CASI Pharmaceuticals Announces Up to $20 Million Convertible Note Financing |
|
12/08/2025 |
News Article |
CASI Pharmaceuticals Announces Results from CID-103 Immune Thrombocytopenia Study at the 67th American Society of Hematology (ASH) Annual Meeting |
|
11/10/2025 |
News Article |
CASI Pharmaceuticals Receives and Appeals Delisting Determination from NASDAQ |


