Product Description
a new generation broad-spectrum intravenous cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Ceftobiprole exhibits potent in vitro activity against a number of Gram-positive and Gram-negative pathogens associated with hospital-acquired pneumonia (HAP) and community-acquired pneumonia (CAP). (Sourced from: https://pubmed.ncbi.nlm.nih.gov/25117196/)
Mechanisms of Action: PBP Binder
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Intravenous
FDA Designation: *
Approval Status: Approved
Approved Countries: Austria | Colombia | France
Approved Indications: None
Known Adverse Events: None
Company: Basilea Pharmaceutica
Company Location:
Company Founding Year: 2000
Additional Commercial Interests: None
Clinical Description
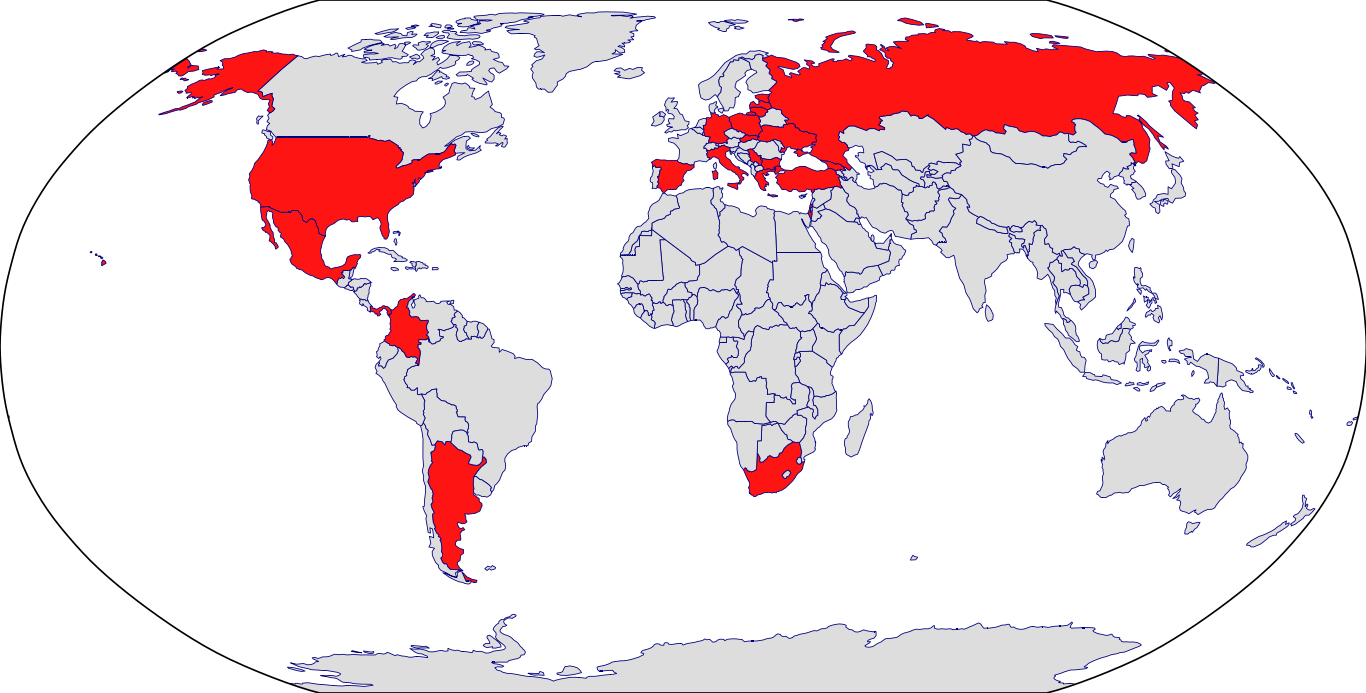
Countries in Clinic: Bulgaria, Estonia, Germany, Latvia, Lithuania, Poland, Slovakia, United States
Active Clinical Trial Count: 2
Recent & Upcoming Milestones
Highest Development Phases
Phase 3: Neonatal Sepsis|Sepsis
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
2022-001837-35 |
2022-001837-35 | P3 |
Completed |
Sepsis |
2024-12-18 |
2025-06-19 |
Treatments |
|
NCT05856227 |
BPR-PIP-003 | P3 |
Completed |
Neonatal Sepsis |
2024-12-18 |
9% |
2024-12-21 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments|Trial Status |


