Product Description
NIO-752 is a drug candidate from Novartis Pharmaceutical for use in patients with progressive supranuclear palsy (PSP). (Sourced from: https://clinicaltrials.gov/ct2/show/NCT04539041)
Mechanisms of Action: Tau Antagonist
Novel Mechanism: Yes
Modality: Antibody Drug Conjugate
Route of Administration: Injection
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: Novartis
Company Location: Europe
Company CEO: Vasant Narasimhan
Additional Commercial Interests: None
Clinical Description
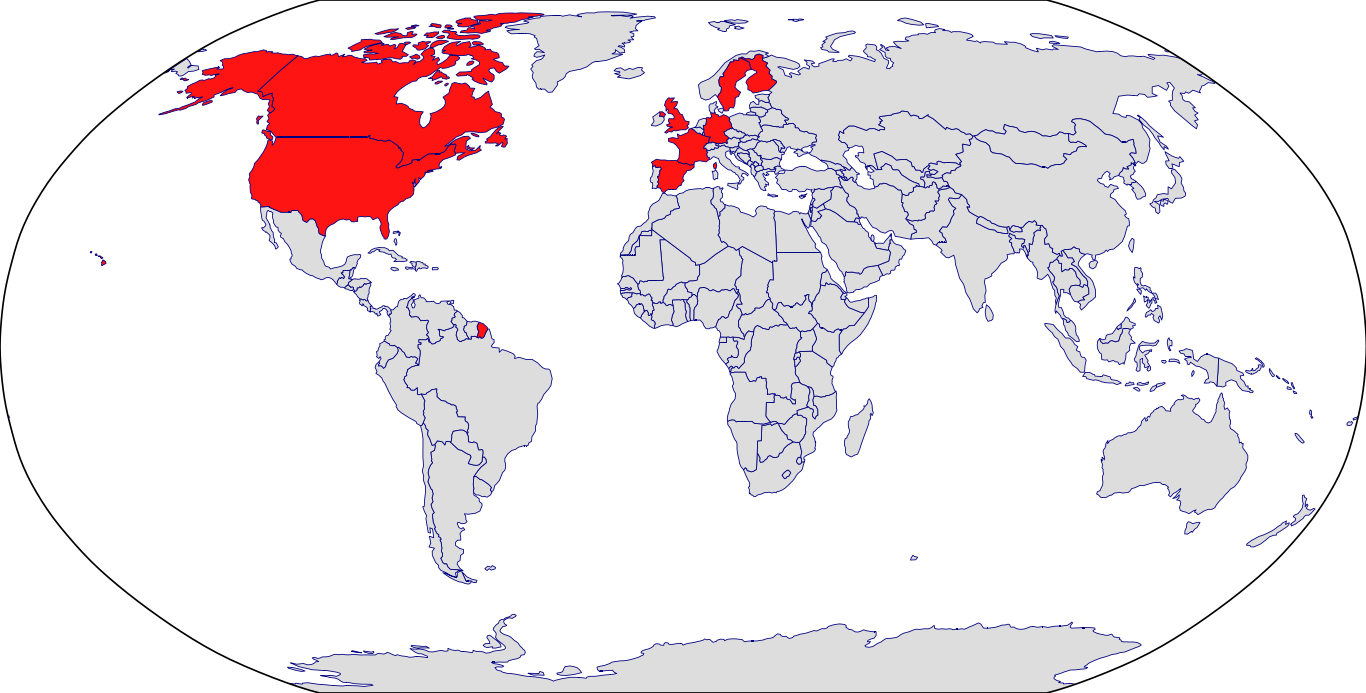
Countries in Clinic: Canada, Finland, France, Germany, Spain, Sweden, United Kingdom, United States
Active Clinical Trial Count: 3
Recent & Upcoming Milestones
Highest Development Phases
Phase 1: Alzheimer Disease|Arthrogryposis|Brain Diseases|Cognitive Dysfunction|Memory Disorders|Paralysis|Progressive Supranuclear Palsy|Supranuclear Palsy, Progressive|Tauopathies
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT05469360 |
CNIO752B12201 | P1 |
Recruiting |
Alzheimer Disease|Tauopathies|Memory Disorders|Brain Diseases|Cognitive Dysfunction |
2026-09-11 |
50% |
2025-08-29 |
Patient Enrollment|Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments|Trial Status |
NCT06372821 |
NIO-SILK | P1 |
Recruiting |
Alzheimer Disease|Arthrogryposis |
2026-07-01 |
2025-07-31 |
Primary Completion Date|Primary Endpoints|Start Date|Study Completion Date|Treatments|Trial Status |
|
NCT04539041 |
CNIO752A02101 | P1 |
Completed |
Paralysis|Supranuclear Palsy, Progressive|Progressive Supranuclear Palsy |
2024-10-17 |
4% |
2024-11-21 |
Primary Endpoints|Treatments|Trial Status |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
02/16/2024 |
PubMed |
Prognostic and Predictive Factors in Early Alzheimer's Disease: A Systematic Review. |
|
02/01/2024 |
PubMed |
Posterior cortical atrophy: new insights into treatments and biomarkers for Alzheimer's disease. |


