Product Description
OCU200 is a biologic product candidate in preclinical development for treating severely sight-threatening diseases like Diabetic Macular Edema (DME), Diabetic Retinopathy (DR), and Wet Age-Related Macular Degeneration (Wet-AMD). (Sourced from: https://ir.ocugen.com/news-releases/news-release-details/ocugen-presents-new-preclinical-ocu200-data-association-research)
Mechanisms of Action: VEGF Binder
Novel Mechanism: Yes
Modality: Fusion Protein
Route of Administration: Intravenous
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: Ocugen
Company Location: Eastern America
Company Founding Year: 2013
Additional Commercial Interests: None
Clinical Description
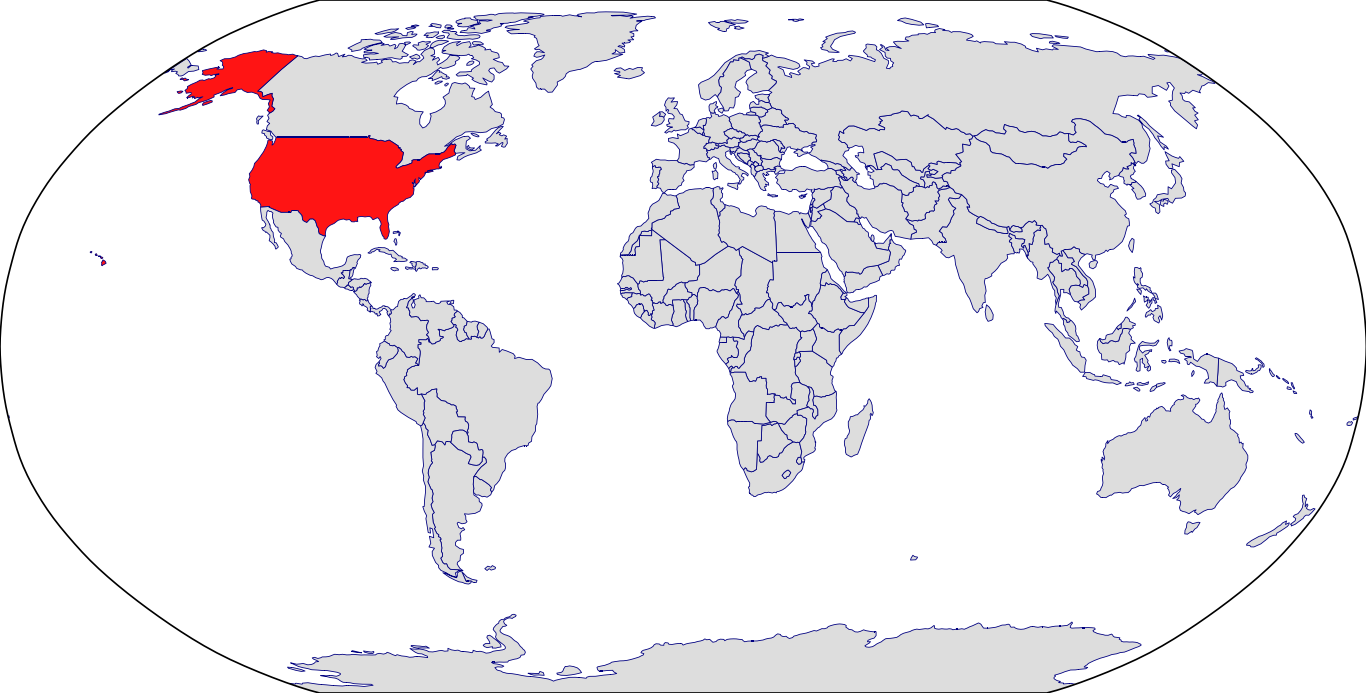
Countries in Clinic: United States
Active Clinical Trial Count: 1
Recent & Upcoming Milestones
Highest Development Phases
Phase 1: Macular Edema
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT05802329 |
DME | P1 |
Recruiting |
Macular Edema |
2026-07-31 |
50% |
2025-12-04 |
Patient Enrollment|Primary Endpoints|Treatments |


