Product Description
AZD-2936 is being developed by AstraZeneca for the treatment of solid tumors. (Sourced from: https://www.astrazeneca.com/our-therapy-areas/pipeline.html)
Mechanisms of Action: PD-1 Inhibitor, TIGIT Inhibitor
Novel Mechanism: Yes
Modality: Bispecific Antibody
Route of Administration: Intravenous
FDA Designation: *
Approval Status: Not Approved
Approved Countries: None
Approved Indications: None
Known Adverse Events: None
Company: AstraZeneca
Company Location: Europe
Company CEO: Pascal Soriot
Additional Commercial Interests: None
Clinical Description
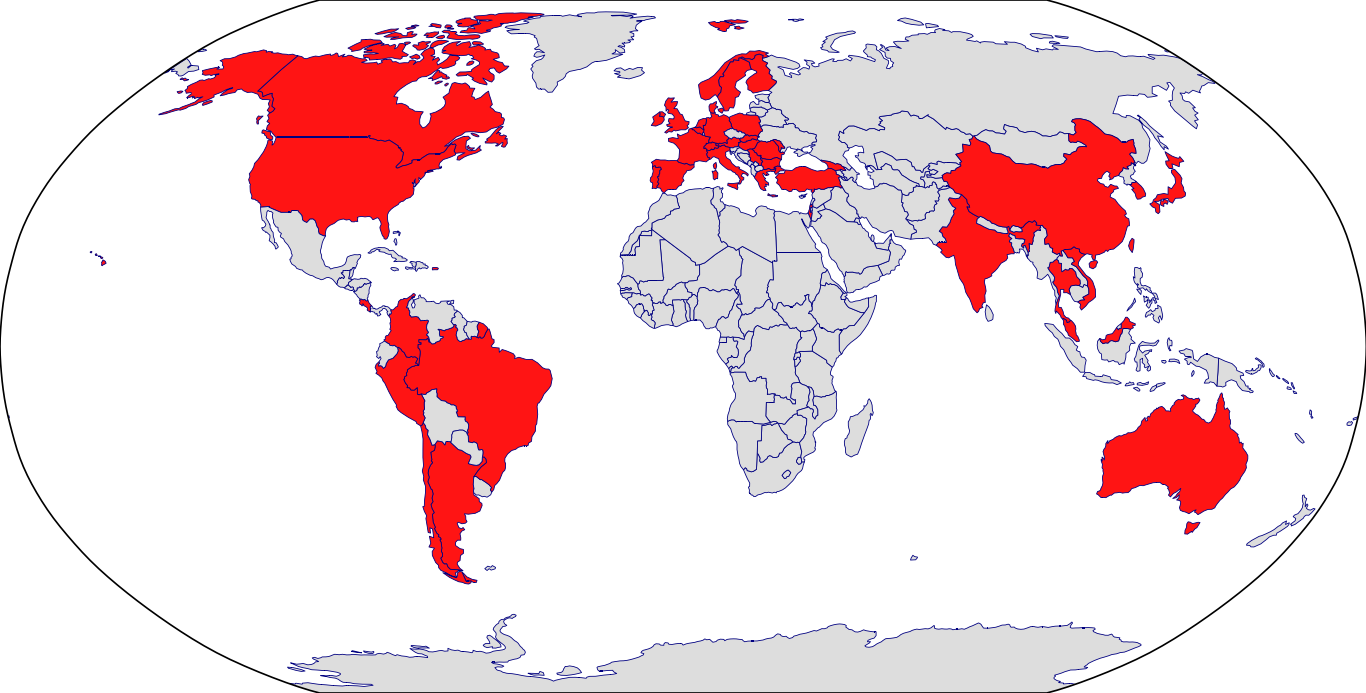
Countries in Clinic: Argentina, Australia, Austria, Belgium, Brazil, Bulgaria, Canada, Chile, China, Colombia, Costa Rica, Czech Republic, Denmark, Finland, France, Georgia, Germany, Greece, Hong Kong, Hungary, India, Ireland, Israel, Italy, Japan, Korea, Malaysia, Moldova, Netherlands, Norway, Peru, Poland, Portugal, Puerto Rico, Romania, Serbia, Singapore, Slovakia, South Korea, Spain, Sweden, Switzerland, Taiwan, Thailand, Turkey, United Kingdom, United States, Unknown Location, Vietnam
Active Clinical Trial Count:
Recent & Upcoming Milestones
- Clinical Outcomes Reported - AstraZeneca presented P3 Oncology Solid Tumor Unspecified results on 2025-05-31 for Rilvegostomig
- Clinical Outcomes Reported - Compugen announced they will present P1 Non-Small-Cell Lung Cancer results in 2H24 for Rilvegostomig
Highest Development Phases
Phase 3: Adenocarcinoma|Bile Duct Cancer|Biliary Tract Cancer|Cholangiocarcinoma|Endometrial Cancer|Esophageal Cancer|Gallbladder Cancer|Gastrointestinal Cancer|Hepatocellular Carcinoma|Lung Cancer|Muscle Cancer|Non-Small-Cell Lung Cancer|Small Cell Lung Cancer|Squamous Cell Carcinoma
Phase 2: Head and Neck Cancer|Oncology Solid Tumor Unspecified
Phase 1: Breast Cancer|Lewy Body Disease|Liver Cancer|Lymphoma|Melanoma|Parkinson's Disease|Sarcoma|Soft Tissue Cancer
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT04541108 |
PBI-MST-01 | P1 |
Recruiting |
Soft Tissue Cancer|Head and Neck Cancer|Liver Cancer|Adenocarcinoma|Lymphoma|Breast Cancer|Melanoma|Sarcoma |
2031-12-01 |
21% |
2024-04-19 |
Primary Endpoints |
NCT07161414 |
ARTEMIDE-subQ | P1 |
Recruiting |
Lewy Body Disease|Parkinson's Disease |
2027-07-19 |
50% |
2025-12-25 |
Primary Completion Date|Primary Endpoints|Start Date|Study Completion Date|Treatments|Trial Status |
jRCT2051250123 |
jRCT2051250123 | P2 |
Recruiting |
Small Cell Lung Cancer|Non-Small-Cell Lung Cancer |
2030-01-08 |
|||
2022-502317-29-00 |
D7987C00001 | P2 |
Recruiting |
Oncology Solid Tumor Unspecified |
2026-11-25 |
2025-05-02 |
Treatments |
|
NCT05775159 |
GEMINI-Hepatobiliary | P2 |
Recruiting |
Hepatocellular Carcinoma|Biliary Tract Cancer |
2026-10-27 |
12% |
2025-11-14 |
|
NCT04995523 |
ARTEMIDE-01 | P2 |
Recruiting |
Non-Small-Cell Lung Cancer |
2026-08-18 |
12% |
2025-06-17 |
Primary Endpoints |
NCT05414032 |
MERIDIAN | P2 |
Recruiting |
Head and Neck Cancer|Squamous Cell Carcinoma |
2026-07-01 |
12% |
2023-10-26 |
Primary Endpoints|Treatments |
2024-510977-27-00 |
D7986C00001 | P2 |
Recruiting |
Gastrointestinal Cancer|Adenocarcinoma|Esophageal Cancer |
2026-03-31 |
2025-05-02 |
Treatments |
|
2024-517780-24-00 |
D702GC00001 | P3 |
Not yet recruiting |
Lung Cancer |
2031-01-25 |
76% |
||
2023-506054-20-00 |
D7025C00001 | P3 |
Recruiting |
Cholangiocarcinoma|Muscle Cancer|Adenocarcinoma|Biliary Tract Cancer|Gallbladder Cancer |
2030-09-30 |
72% |
2025-05-02 |
Treatments |
NCT07221253 |
AB02 | P3 |
Recruiting |
Biliary Tract Cancer|Gallbladder Cancer |
2029-07-04 |
78% |
2026-01-09 |
|
NCT06989112 |
DE-01 | P3 |
Recruiting |
Endometrial Cancer |
2029-01-19 |
72% |
2025-05-28 |
Primary Endpoints |
2023-505077-32-00 |
D7632C00001 | P3 |
Recruiting |
Non-Small-Cell Lung Cancer |
2030-05-24 |
68% |
2025-05-02 |
Treatments |
jRCT2051250018 |
jRCT2051250018 | P3 |
Recruiting |
Hepatocellular Carcinoma |
2030-03-31 |
|||
jRCT2011250015 |
jRCT2011250015 | P3 |
Recruiting |
Endometrial Cancer |
2031-03-31 |
|||
2024-512195-35-00 |
D926TC00001 | P3 |
Not yet recruiting |
Adenocarcinoma|Non-Small-Cell Lung Cancer |
2035-02-01 |
9% |
2025-05-02 |
Treatments |
jRCT2061240062 |
jRCT2061240062 | P3 |
Not yet recruiting |
Non-Small-Cell Lung Cancer |
2035-03-31 |
|||
jRCT2051250117 |
jRCT2051250117 | P3 |
Recruiting |
Non-Small-Cell Lung Cancer|Small Cell Lung Cancer |
2032-01-31 |
|||
jRCT2031250011 |
jRCT2031250011 | P3 |
Recruiting |
Esophageal Cancer|Gastrointestinal Cancer|Adenocarcinoma |
2031-02-28 |
|||
2023-508056-19-00 |
DESTINYEndometrial01 | P3 |
Not yet recruiting |
Endometrial Cancer |
2031-02-24 |
72% |
||
2024-512583-57-00 |
D702AC00001 | P3 |
Not yet recruiting |
Esophageal Cancer|Adenocarcinoma|Gastrointestinal Cancer |
2030-12-09 |
77% |
||
2023-508852-21-00 |
D9077C00001 | P2 |
Recruiting |
Non-Small-Cell Lung Cancer |
2030-12-10 |
12% |
2025-05-02 |
Treatments |
NCT05061550 |
NeoCOAST-2 | P2 |
Recruiting |
Non-Small-Cell Lung Cancer |
2030-05-09 |
12% |
2024-11-23 |
Patient Enrollment|Primary Endpoints |
jRCT2031230509 |
jRCT2031230509 | P3 |
Not yet recruiting |
Biliary Tract Cancer |
2030-09-30 |
|||
jRCT2031240095 |
jRCT2031240095 | P3 |
Recruiting |
Non-Small-Cell Lung Cancer |
2030-06-30 |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
12/17/2025 |
News Article |
Compugen Monetizes Portion of Rilvegostomig Future Royalties to AstraZeneca for Up to $90 Million |
|
11/26/2025 |
News Article |
IMFINZI® approved in the US as first and only perioperative immunotherapy for patients with early gastric and gastroesophageal cancers |
|
11/04/2025 |
News Article |
Compugen to Participate in Stifel 2025 Healthcare Conference |
|
10/27/2025 |
News Article |
Compugen to Release Third Quarter 2025 Results on Monday, November 10, 2025 |


