Product Description
The glucagon-like peptide-1 receptor agonist (GLP-1RA) semaglutide is the most recently approved agent of this drug class, and the only GLP-1RA currently available as both subcutaneous and oral formulation. (Sourced from: https://pubmed.ncbi.nlm.nih.gov/34305810/)
Mechanisms of Action: GLP-1 Agonist
Novel Mechanism: No
Modality: Peptide/Protein
Route of Administration: Oral, Subcutaneous
FDA Designation: Priority Review - Non-alcoholic Steatohepatitis *
Approval Status: Approved
Approved Countries: Argentina | Australia | Austria | Bangladesh | Belgium | Bosnia | Brazil | Canada | Chile | Colombia | Croatia | Cyprus | Czech | Denmark | Dominican Republic | Ecuador | Egypt | Estonia | European Medicines Agency | Finland | France | Germany | Greece | Hong Kong | Hungary | Iceland | India | Indonesia | Ireland | Israel | Italy | Japan | Jordan | Korea | Latvia | Lebanon | Lithuania | Luxembourg | Malaysia | Mexico | Netherlands | New Zealand | Norway | Peru | Philippines | Poland | Portugal | Romania | Russia | Saudi Arabia | Serbia | Singapore | Slovakia | Slovenia | Spain | Sweden | Switzerland | Taiwan | Turkey | Ukraine | United Arab Emirates | United Kingdom | United States
Approved Indications: None
Known Adverse Events: None
Company: Novo Nordisk
Company Location: Europe
Company Founding Year: 1923
Additional Commercial Interests: None
Clinical Description
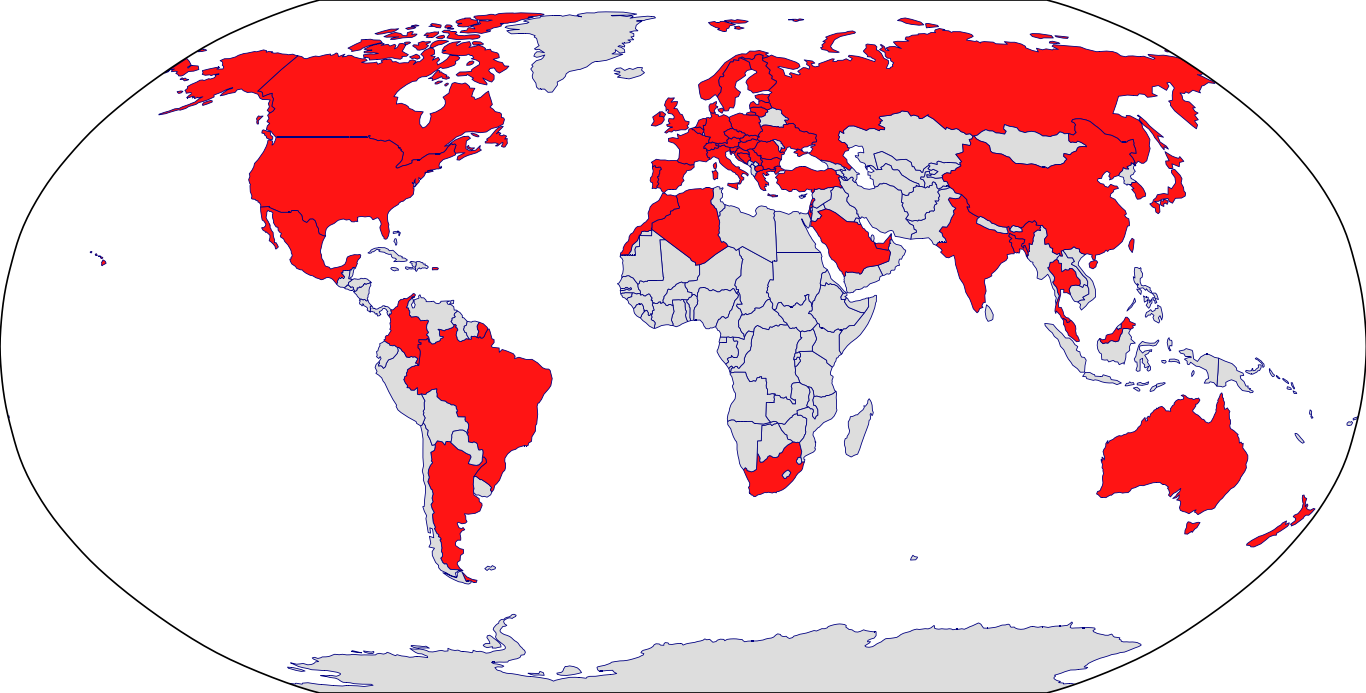
Countries in Clinic: Algeria, Argentina, Australia, Austria, Bangladesh, Belgium, Brazil, Bulgaria, Canada, China, Colombia, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hong Kong, Hungary, India, Ireland, Israel, Italy, Japan, Korea, Latvia, Lebanon, Malaysia, Mexico, Morocco, Netherlands, New Zealand, North Macedonia, Norway, Poland, Portugal, Puerto Rico, Romania, Russia, Saudi Arabia, Serbia, Singapore, Slovakia, Slovenia, South Africa, South Korea, Spain, Sweden, Switzerland, Taiwan, Thailand, Turkey, Ukraine, United Kingdom, United States, Unknown Location
Active Clinical Trial Count: 173
Recent & Upcoming Milestones
- Clinical Outcomes Reported - Novo Nordisk presented P3 Alzheimer Disease results on 2025-11-24 for Semaglutide
- Clinical Outcomes Reported - Novo Nordisk presented P3 Obesity|Weight Loss results on 2025-11-05 for Semaglutide
- Clinical Outcomes Reported - Novo Nordisk presented P2 Non-alcoholic Steatohepatitis results on 2025-11-09 for Semaglutide
Highest Development Phases
Phase 3: Albuminuria|Atherosclerosis|Diabetes, Gestational|Diabetic Retinopathy|Dyslipidemia|Fatty Liver, Alcoholic|Glucose Intolerance|Heart Failure|Heart Failure, Chronic|Heart Failure, Diastolic|Hepatitis, Alcoholic|Hypercholesterolemia|Hypertension|Ischemic Stroke|Kidney Diseases|Kidney Failure, Chronic|Non-alcoholic Steatohepatitis|Obesity|Osteoarthritis, Knee|Overweight|Peripheral Arterial Disease|Peripheral Vascular Diseases|Prediabetic State|Stomach Diseases|Stroke|Type 1 Diabetes|Type 2 Diabetes|Weight Loss
Phase 2: Diabetic Nephropathy|Inflammation|Mucositis|Muscular Atrophy|Neuropathic Pain|Prediabetes|Schizophrenia|Weight Gain
Phase 1: Healthy Volunteers|Hyperglycemia|Myocardial Infarction|Obesity, Morbid|Pain Unspecified|Polycystic Ovary Syndrome|Sleep Apnea, Obstructive
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT07430059 |
SLIM-1 | P1 |
Not yet recruiting |
Overweight|Obesity |
2026-08-01 |
88% |
2026-02-25 |
Primary Endpoints|Treatments |
NCT06222437 |
046.OBG.2023.D | P1 |
Not yet recruiting |
Polycystic Ovary Syndrome |
2026-01-01 |
2024-03-28 |
||
NCT06403761 |
NN9388-7782 | P1 |
Completed |
Type 2 Diabetes |
2025-12-28 |
88% |
2026-02-28 |
Patient Enrollment|Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments|Trial Status |
NCT07092605 |
ALRx010 | P1 |
Enrolling by invitation |
Inflammation |
2025-11-01 |
12% |
2025-07-31 |
Primary Endpoints|Treatments |
CTR20251083 |
CTR20251083 | P1 |
Completed |
Stroke|Myocardial Infarction|Type 2 Diabetes |
2025-06-30 |
2025-07-15 |
Primary Completion Date|Study Completion Date|Treatments|Trial Status |
|
NCT05784402 |
NN9924-4891 | P1 |
Completed |
Pain Unspecified|Healthy Volunteers |
2024-02-23 |
50% |
2024-05-02 |
Patient Enrollment|Primary Endpoints|Study Completion Date|Treatments|Trial Status |
NCT07101939 |
PROACT | P2 |
Recruiting |
Obesity|Muscular Atrophy |
2027-03-01 |
12% |
2025-12-20 |
Patient Enrollment|Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments |
NCT06449625 |
PROTECT | P2 |
Not yet recruiting |
Inflammation|Mucositis |
2026-12-31 |
12% |
2024-06-11 |
|
2023-503753-35-01 |
FIT-HF trial | P2 |
Recruiting |
Obesity|Heart Failure |
2026-12-31 |
2025-05-02 |
Treatments |
|
2023-509662-38-00 |
NN9388-7864 | P2 |
Recruiting |
Type 2 Diabetes|Neuropathic Pain |
2026-06-23 |
|||
NCT05822609 |
RT1D | P2 |
Recruiting |
Type 1 Diabetes|Kidney Diseases|Diabetic Nephropathy |
2026-06-01 |
2024-06-07 |
Primary Endpoints|Start Date|Treatments|Trial Status |
|
NCT05424003 |
HM20024306 | P2 |
Recruiting |
Weight Gain |
2026-02-01 |
50% |
2024-04-10 |
Primary Endpoints |
NCT06131372 |
NN9388-7700 | P2 |
Completed |
Type 2 Diabetes|Obesity|Kidney Failure, Chronic |
2025-09-24 |
50% |
2025-11-29 |
|
2020-004374-22 |
HISTORI - Home-based Intervention with Semaglutide Treatment Of | P2 |
Active, not recruiting |
Schizophrenia|Prediabetes |
2023-06-16 |
2022-03-13 |
Treatments |
|
NCT04822181 |
ESSENCE | P3 |
Active, not recruiting |
Fatty Liver, Alcoholic|Non-alcoholic Steatohepatitis|Hepatitis, Alcoholic |
2029-04-25 |
52% |
2025-03-04 |
Patient Enrollment|Primary Endpoints|Treatments |
NCT05569772 |
SERENA | P3 |
Recruiting |
Glucose Intolerance|Diabetes, Gestational |
2028-07-01 |
2023-09-19 |
Primary Endpoints|Start Date |
|
2025-522326-13-00 |
2025-522326-13-00 | P3 |
Not yet recruiting |
Atherosclerosis|Hypertension|Hypercholesterolemia|Dyslipidemia|Ischemic Stroke|Albuminuria|Obesity|Type 1 Diabetes |
2028-06-01 |
|||
2023-506827-26-00 |
NN9535-4352 | P3 |
Active, not recruiting |
Type 2 Diabetes|Diabetic Retinopathy |
2027-11-07 |
2025-05-02 |
Treatments |
|
NCT05669755 |
REDEFINE 3 | P3 |
Active, not recruiting |
Stroke|Stomach Diseases |
2027-09-01 |
53% |
2025-10-23 |
Patient Enrollment|Primary Endpoints|Treatments |
NCT06633783 |
JY29-2-302 | P3 |
Not yet recruiting |
Weight Loss|Obesity |
2026-05-30 |
21% |
||
NCT06604624 |
HD1916-003 | P3 |
Active, not recruiting |
Obesity |
2026-02-01 |
40% |
2024-11-13 |
Patient Enrollment|Primary Endpoints|Start Date|Treatments|Trial Status |
NCT04777409 |
EVOKE Plus | P3 |
Active, not recruiting |
Alzheimer Disease |
2025-09-15 |
67% |
2025-12-03 |
|
NCT04560998 |
STRIDE | P3 |
Completed |
Type 2 Diabetes|Peripheral Vascular Diseases|Peripheral Arterial Disease |
2024-06-05 |
75% |
2024-08-21 |
|
NCT04916470 |
STEP HFpEF DM | P3 |
Completed |
Heart Failure, Diastolic|Heart Failure, Chronic|Type 2 Diabetes |
2023-10-11 |
99% |
2023-11-01 |
|
NCT05064735 |
NN9536-4578 | P3 |
Completed |
Obesity|Osteoarthritis, Knee |
2023-07-24 |
62% |
2023-09-15 |
Primary Endpoints|Study Completion Date|Treatments|Trial Status |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
03/11/2026 |
News Article |
GoodRx Partners with Viatris to Offer Up to 85% Savings on Established Brand Medications |
|
03/10/2026 |
News Article |
NorthStrive Biosciences Announces Launch of EL-32 Preclinical Study Evaluating Muscle Preservation in GLP-1 Weight Loss Therapy |
|
03/10/2026 |
News Article |
Hoth Therapeutics Reports Positive Female Preclinical Data Showing HT-VA Restores Cholesterol Levels and Improves Lipid Metabolism in MASLD Model |
|
03/09/2026 |
News Article |
Wellgistics Targets $70B Market Breakthrough Addressing Ozempic / GLP-1 Muscle Loss with Forzet(TM) |


