Product Description
For Retinal Vasculopathy Cerebral Leukoencephalopathy; crizanlizumab is a humanized monoclonal anti-P-selectin antibody that prevents leukocyte adhesion to the vascular endothelium, thereby limiting risk of microvascular occlusion (Sourced from: https://clinicaltrials.gov/ct2/show/NCT04611880)
Mechanisms of Action: P-selectin Inhibitor
Novel Mechanism: No
Modality: Antibody
Route of Administration: Intravenous
FDA Designation: *
Approval Status: Approved
Approved Countries: Australia | Austria | Belgium | Brazil | Colombia | Croatia | Cyprus | Czech | Denmark | Estonia | European Medicines Agency | Finland | France | Germany | Greece | Hungary | Iceland | Ireland | Israel | Italy | Jordan | Latvia | Lebanon | Lithuania | Luxembourg | Netherlands | Norway | Poland | Portugal | Saudi Arabia | Slovakia | Spain | Sweden | United Arab Emirates | United Kingdom | United States
Approved Indications: None
Known Adverse Events: None
Company: Novartis
Company Location: Europe
Company Founding Year: 1996
Additional Commercial Interests: None
Clinical Description
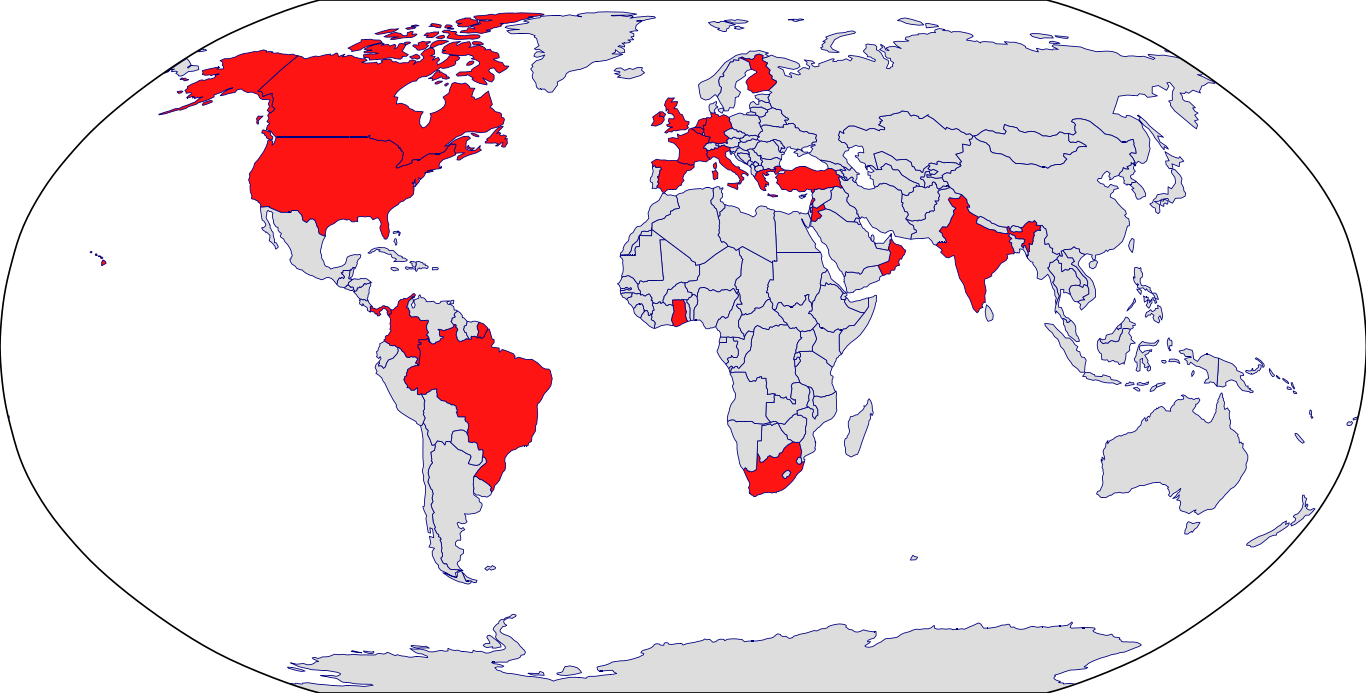
Countries in Clinic: Belgium, Brazil, Canada, Colombia, Finland, France, Germany, Ghana, Greece, India, Ireland, Italy, Jordan, Lebanon, Netherlands, Oman, Panama, South Africa, Spain, Turkey, United Kingdom, United States, Unknown Location
Active Clinical Trial Count: 9
Recent & Upcoming Milestones
Highest Development Phases
Phase 3: Anemia, Sickle Cell|Sickle Cell Trait
Phase 2: Kidney Failure, Chronic|Priapism
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT03814746 |
STAND | P3 |
Active, not recruiting |
Anemia, Sickle Cell|Sickle Cell Trait |
2022-08-31 |
24% |
2025-09-25 |
Patient Enrollment|Primary Endpoints|Treatments |
NCT03938454 |
SPARTAN | P2 |
Completed |
Anemia, Sickle Cell|Priapism |
2023-03-28 |
50% |
2025-03-11 |
Primary Endpoints|Treatments |
NCT04053764 |
STEADFAST | P2 |
Completed |
Kidney Failure, Chronic|Anemia, Sickle Cell |
2023-03-20 |
12% |
2023-02-18 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments |
NCT05469828 |
SEG101 | P2 |
Not yet recruiting |
Anemia, Sickle Cell |
2028-07-01 |
12% |
2023-08-31 |
Primary Completion Date|Primary Endpoints|Start Date|Study Completion Date|Treatments |
2023-508689-14-00 |
CSEG101A2301 | P3 |
Recruiting |
Anemia, Sickle Cell |
2026-12-14 |
2025-05-02 |
Treatments |
|
2020-003601-66 |
SMART study | P3 |
Active, not recruiting |
Anemia, Sickle Cell |
2023-07-14 |
2022-03-13 |
Treatments |
|
NCT03474965 |
CSEG101B2201 | P2 |
Completed |
Anemia, Sickle Cell |
2024-11-06 |
50% |
2025-07-22 |
Primary Endpoints |
NCT03264989 |
CSEG101A2202 | P2 |
Completed |
Anemia, Sickle Cell |
2023-06-26 |
50% |
2024-04-24 |
Primary Endpoints|Treatments |
NCT06439082 |
SPARKLE | P3 |
Recruiting |
Anemia, Sickle Cell |
2029-03-23 |
24% |
2024-11-14 |


