Product Description
Tirzepatide is a novel investigational once-weekly dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that integrates the actions of both incretins into a single molecule, representing a new class of medicines being studied for the treatment of type 2 diabetes. (Sourced from: https://investor.lilly.com/news-releases/news-release-details/tirzepatide-results-published-lancet-show-superior-a1c-and-body)
Mechanisms of Action: GLP-1 Agonist, GIP Agonist
Novel Mechanism: No
Modality: Peptide/Protein
Route of Administration: Subcutaneous
FDA Designation: Fast Track - Obesity|Obesity, Morbid|Overweight *
Approval Status: Approved
Approved Countries: Australia | Austria | Belgium | Croatia | Czech | Estonia | European Medicines Agency | Finland | Germany | Greece | Hungary | Iceland | Ireland | Israel | Italy | Japan | Latvia | Lithuania | Poland | Portugal | Romania | Saudi Arabia | Slovakia | Spain | Sweden | Switzerland | United Arab Emirates | United Kingdom | United States
Approved Indications: None
Known Adverse Events: None
Company: Eli Lilly
Company Location: Eastern America
Company Founding Year: 1876
Additional Commercial Interests: None
Clinical Description
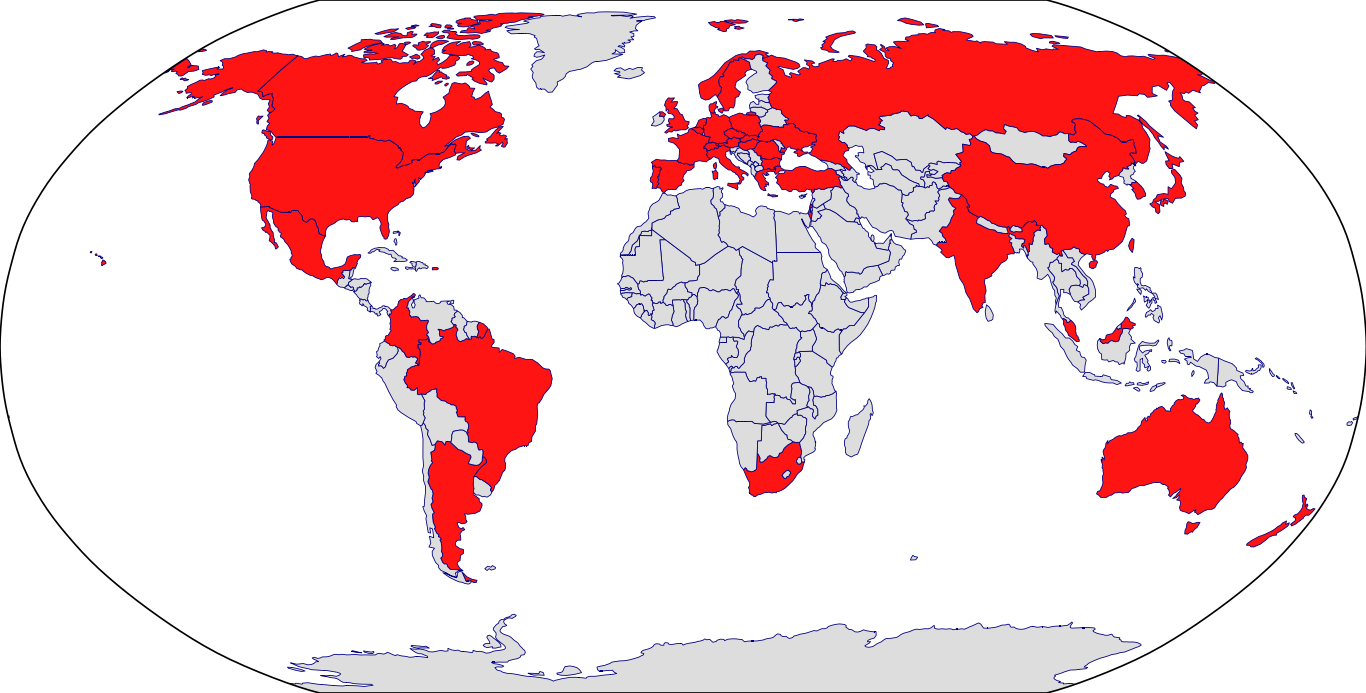
Countries in Clinic: Argentina, Australia, Austria, Belgium, Brazil, Bulgaria, Canada, China, Colombia, Czech Republic, Denmark, France, Germany, Greece, Hungary, India, Israel, Italy, Japan, Korea, Malaysia, Mexico, Netherlands, New Zealand, Norway, Poland, Portugal, Puerto Rico, Romania, Russia, Singapore, Slovakia, South Africa, South Korea, Spain, Sweden, Switzerland, Taiwan, Turkey, Ukraine, United Kingdom, United States, Unknown Location
Active Clinical Trial Count: 75
Recent & Upcoming Milestones
- Clinical Outcomes Reported - Eli Lilly presented P3 Obesity results on 2026-02-18 for Tirzepatide
- Clinical Outcomes Reported - Eli Lilly presented P3 Type 2 Diabetes|Cardiovascular results on 2025-07-30 for Tirzepatide
- Clinical Outcomes Reported - Eli Lilly presented P3 Obesity results on 2025-06-20 for Tirzepatide
Highest Development Phases
Phase 3: Apnea|Arthritis, Psoriatic|Colitis, Ulcerative|Crohn Disease|Fatty Liver|Fatty Liver, Alcoholic|Glucose Metabolism Disorders|Heart Failure, Chronic|Heart Failure, Diastolic|Non-alcoholic Fatty Liver Disease|Obesity|Overweight|Prediabetic State|Psoriasis|Sleep Apnea, Obstructive|Type 1 Diabetes|Type 2 Diabetes|Weight Gain|Weight Loss
Phase 2: Binge-Eating Disorder|Hepatitis, Alcoholic|Kidney Diseases|Kidney Failure, Chronic|Non-alcoholic Steatohepatitis|Obesity, Morbid|Protein Deficiency
Phase 1: General Diabetes|Healthy Volunteers|Pediatric Obesity
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
ACTRN12626000224325 |
ACTRN12626000224325 | P1 |
Not yet recruiting |
General Diabetes|Healthy Volunteers |
2026-03-18 |
2026-02-24 |
Treatments |
|
2024-000081-22 |
A Study of Tirzepatide (LY3298176) in Pediatric Participants With Obesity | P1 |
Active, not recruiting |
Pediatric Obesity |
2007-09-15 |
2025-07-09 |
Primary Completion Date|Start Date|Study Completion Date|Treatments|Trial Status |
|
NCT06847399 |
LIBERATE | P2 |
Recruiting |
Overweight|Obesity|Binge-Eating Disorder |
2027-12-01 |
12% |
2025-09-23 |
Primary Endpoints|Start Date|Treatments|Trial Status |
2023-506082-60-00 |
I8F-MC-GPIG | P2 |
Recruiting |
Overweight|Obesity|Kidney Diseases|Type 2 Diabetes |
2026-12-08 |
12% |
2025-05-02 |
Treatments |
NCT05536804 |
TREASURE-CKD | P2 |
Active, not recruiting |
Overweight|Obesity|Type 2 Diabetes|Kidney Failure, Chronic |
2026-09-01 |
12% |
2025-07-25 |
Primary Endpoints|Treatments|Trial Status |
NCT06965413 |
GYMINDA | P2 |
Active, not recruiting |
Overweight|Obesity, Morbid |
2026-08-24 |
12% |
2026-02-13 |
Primary Completion Date|Primary Endpoints|Study Completion Date |
NCT06643728 |
J4Z-MC-GIDF | P2 |
Active, not recruiting |
Overweight|Protein Deficiency|Obesity |
2026-04-01 |
12% |
2025-09-27 |
Patient Enrollment|Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments|Trial Status |
NCT04166773 |
SYNERGY-NASH | P2 |
Completed |
Hepatitis, Alcoholic|Fatty Liver, Alcoholic|Non-alcoholic Steatohepatitis |
2023-12-11 |
82% |
2025-01-25 |
Patient Enrollment|Primary Endpoints|Treatments |
jRCT2031220154 |
jRCT2031220154 | P3 |
Recruiting |
Obesity|Apnea |
2024-05-31 |
|||
NCT05412004 |
SURMOUNT-OSA | P3 |
Completed |
Sleep Apnea, Obstructive|Obesity |
2024-03-12 |
74% |
2025-05-01 |
Primary Endpoints |
jRCT2021250031 |
jRCT2021250031 | P3 |
Not yet recruiting |
Fatty Liver |
2030-10-31 |
|||
NCT07165028 |
SYNERGY-Outcomes | P3 |
Recruiting |
Non-alcoholic Fatty Liver Disease|Fatty Liver, Alcoholic |
2030-08-01 |
40% |
2025-11-06 |
Primary Endpoints|Start Date|Trial Status |
NCT06937099 |
COMMIT-CD | P3 |
Recruiting |
Overweight|Crohn Disease|Obesity |
2028-05-01 |
30% |
2025-08-27 |
Primary Endpoints|Treatments |
NCT06937086 |
COMMIT-UC | P3 |
Recruiting |
Obesity|Colitis, Ulcerative|Overweight |
2028-04-01 |
30% |
2025-08-27 |
Primary Endpoints |
NCT06439277 |
SURMOUNT-ADOLESCENTS-2 | P3 |
Recruiting |
Obesity|Weight Gain |
2027-10-01 |
28% |
2025-08-27 |
Primary Endpoints|Treatments |
NCT06630585 |
AID-JUNCT | P3 |
Recruiting |
Type 1 Diabetes |
2026-06-01 |
2025-06-07 |
||
NCT06047548 |
SURMOUNT-MAINTAIN | P3 |
Active, not recruiting |
Obesity|Overweight|Weight Loss |
2026-05-01 |
85% |
2025-01-08 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments |
NCT06221969 |
NN9388-4894 | P3 |
Active, not recruiting |
Type 2 Diabetes |
2026-02-20 |
63% |
2025-04-09 |
Primary Completion Date|Primary Endpoints |
NCT06588283 |
TOGETHER-PsO | P3 |
Active, not recruiting |
Psoriasis|Obesity |
2026-01-08 |
24% |
2026-01-21 |
Primary Completion Date|Primary Endpoints|Treatments |
NCT06588296 |
TOGETHER-PsA | P3 |
Active, not recruiting |
Arthritis, Psoriatic|Obesity |
2025-11-18 |
24% |
2026-01-22 |
Patient Enrollment|Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments |
NCT05822830 |
SURMOUNT-5 | P3 |
Completed |
Obesity|Overweight|Prediabetic State |
2024-11-13 |
31% |
2025-11-27 |
Primary Endpoints|Treatments |
NCT05260021 |
SURPASS-PEDS | P3 |
Completed |
Type 2 Diabetes|Glucose Metabolism Disorders |
2024-07-30 |
75% |
2025-02-28 |
|
NCT04847557 |
The SUMMIT Trial | P3 |
Completed |
Heart Failure, Chronic|Heart Failure, Diastolic|Obesity |
2024-07-02 |
18% |
2024-07-23 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Trial Status |
NCT05912621 |
Tirzepatide | P2 |
Recruiting |
Overweight|Obesity |
2028-06-01 |
50% |
2024-12-05 |
Primary Endpoints |
2025-522674-36-00 |
N1T-MC-MALO | P3 |
Not yet recruiting |
Unknown |
2029-10-11 |
40% |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
03/09/2026 |
News Article |
TrimRx Telehealth Weight Loss Platform Examined in 2026 Consumer Research Report on GLP-1 Medication Access, Pricing Transparency and Safety Considerations |
|
03/09/2026 |
News Article |
Veru Enrolls First Patient in Phase 2b PLATEAU Clinical Trial of Enobosarm and Semaglutide Combination for High Quality Weight Loss |
|
03/09/2026 |
News Article |
Wellgistics Targets $70B Market Breakthrough Addressing Ozempic / GLP-1 Muscle Loss with Forzet(TM) |
|
03/06/2026 |
News Article |
GoodRx to Expand Employer-Sponsored Access to Zepbound® KwikPen® |


