Product Description
Trilaciclib is being evaluated across a range of tumor types and chemotherapy regimens to assess its potential myeloprotection, antitumor efficacy and safety in combination with chemotherapy and other agents. (Sourced from: https://www.g1therapeutics.com/pipeline/trilaciclib/)
Mechanisms of Action: CDK Inhibitor
Novel Mechanism: No
Modality: Small Molecule
Route of Administration: Intravenous
FDA Designation: Fast Track - Breast Cancer|Oncology Solid Tumor Unspecified|Triple Negative Breast Cancer *
Approval Status: Approved
Approved Countries: United States
Approved Indications: None
Known Adverse Events: None
Company: Pharmacosmos
Company Location: Europe
Company CEO:
Additional Commercial Interests: None
Clinical Description
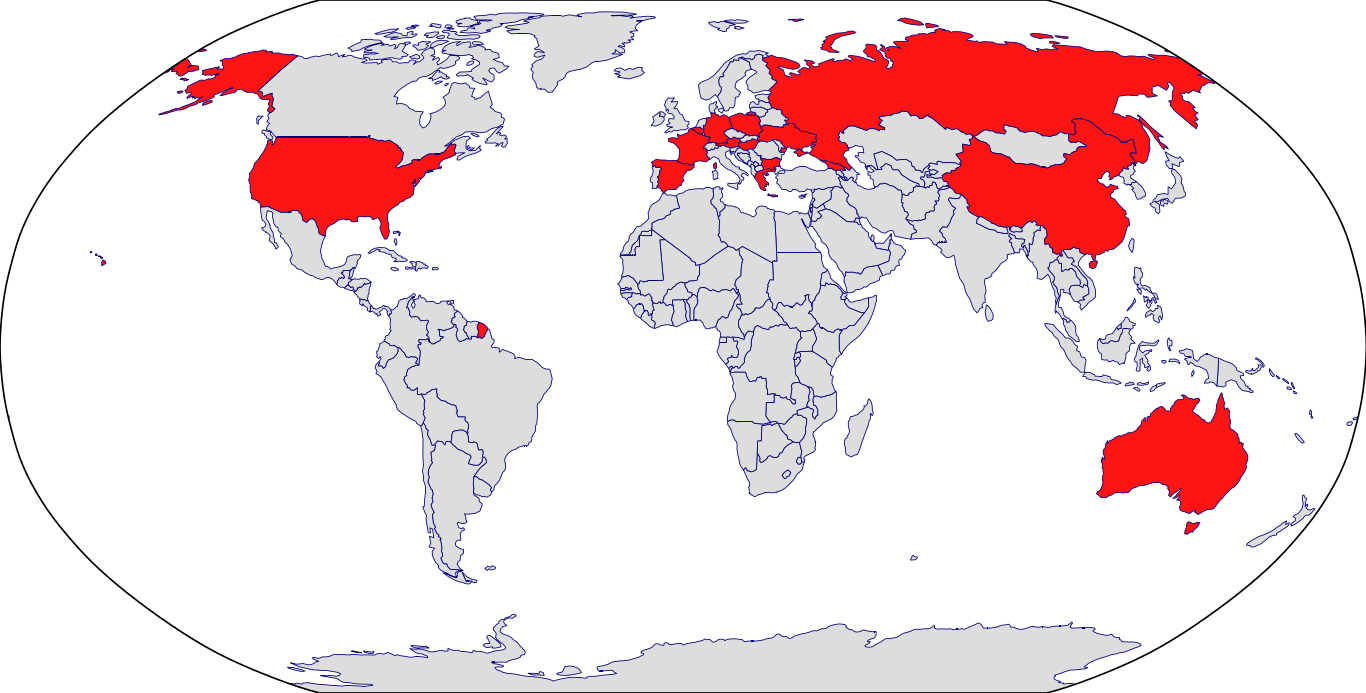
Countries in Clinic: Australia, Austria, Belgium, Bulgaria, China, France, Georgia, Germany, Greece, Hungary, Moldova, Poland, Russia, Spain, Ukraine, United States
Active Clinical Trial Count: 8
Recent & Upcoming Milestones
- Clinical Outcomes Reported - G1 presented P3 Triple Negative Breast Cancer results on 2024-06-24 for Trilaciclib
- Clinical Outcomes Reported - G1 presented P2 Triple Negative Breast Cancer results on 2024-06-02 for Trilaciclib
- Clinical Outcomes Reported - G1 presented P2 Breast Cancer|Oncology Solid Tumor Unspecified|Triple Negative Breast Cancer results on 2024-06-02 for Trilaciclib
Highest Development Phases
Phase 3: Bone Cancer|Small Cell Lung Cancer|Triple Negative Breast Cancer
Phase 2: Non-Small-Cell Lung Cancer
Trial ID |
Trial |
Phase |
Trial Status |
Disease |
Primary Completion Date |
Probability of Success |
Latest Trial Update Date |
Data Updated |
|---|---|---|---|---|---|---|---|---|
NCT06027268 |
ToPCourT | P2 |
Active, not recruiting |
Triple Negative Breast Cancer |
2026-09-01 |
12% |
2025-10-23 |
Primary Endpoints |
NCT06370416 |
HSKY002 | P2 |
Enrolling by invitation |
Non-Small-Cell Lung Cancer |
2026-01-30 |
12% |
2026-01-29 |
Patient Enrollment|Primary Completion Date|Primary Endpoints|Start Date|Study Completion Date|Treatments|Trial Status |
NCT05578326 |
LCCC2117 | P2 |
Recruiting |
Small Cell Lung Cancer |
2025-12-25 |
12% |
2024-06-28 |
Primary Completion Date|Primary Endpoints|Study Completion Date|Treatments |
NCT06698965 |
IS24047 | P2 |
Recruiting |
Small Cell Lung Cancer |
2029-10-31 |
40% |
2024-11-27 |
|
2022-502357-34-00 |
G1T28-211 | P3 |
Recruiting |
Small Cell Lung Cancer |
2027-05-03 |
2025-05-02 |
Treatments |
|
NCT04799249 |
PRESERVE 2 | P3 |
Completed |
Triple Negative Breast Cancer |
2024-05-24 |
7% |
2024-08-02 |
Primary Completion Date|Primary Endpoints|Start Date|Study Completion Date|Trial Status |
2020-004930-39 |
2020-004930-39 | P3 |
Completed |
Triple Negative Breast Cancer |
2024-04-24 |
7% |
2025-07-07 |
Treatments |
CTR20210562 |
CTR20210562 | P3 |
Active, not recruiting |
Small Cell Lung Cancer|Bone Cancer |
None |
2025-04-29 |
Patient Enrollment|Treatments |
Recent News Events
Date |
Type |
Title |
|---|---|---|
|
12/22/2025 |
News Article |
Ipsen expands early development pipeline with Simcere Zaiming's innovative antibody drug conjugate |
|
11/06/2025 |
News Article |
Hatteras Venture Partners Honors HistoSonics with the Charles A. Sanders Award for Entrepreneurial Excellence |
|
10/16/2025 |
News Article |
NextCure and Simcere Zaiming Announce Expansion of Ongoing Phase 1 Trial of SIM0505 (CDH6 ADC) into the United States |
|
08/20/2025 |
News Article |
BioGene Therapeutics Inc. Appoints Dr. Francis Tavares, PhD., as Chief Technology Officer |


